|
Activity:
Herbicide
(amide; triazole)
CAS Name:
1-[4-chloro-3-[(2,2,3,3,3-pentafluoropropoxy)methyl]phenyl]-5-phenyl-1H-1,2,4-triazole-3-carboxamide
Strucuture:
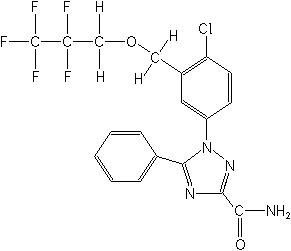
Regulatory
Information
(only comprehensive for the US) |
| US
EPA Registered: |
No |
| European
Commission: |
Not
allowed to be used as an active ingredient after July 25,
2003. |
| Other
Information |
| Molecular
Formula: |
C19H14Cl
F5 N4O2 |
| Entry
Year: |
1989 |
| Inventing
Company: |
Kureha |
| Manufacturers: |
Monsanto |
| Other
Names: |
KNW-739
MON-18500
Ovation
Quatatim |
| Of
special interest: |
| PAN
Data |
| November
26, 2002 -
European Commission: Flupoxam is one of 320 pesticides
to be withdrawn in July 2003.
"Some 320 substances used in plant
protection products (PPPs) – including insecticides, fungicides
and herbicides – are to be withdrawn from the market by 25 July
2003 as part of the European Commission’s new approach to the
evaluation of active substances in plant protection products.
This aims to improve safeguards to ensure that all such products
in use are safe for the environment and human health. Users,
wholesalers and retailers of plant protection products will
need to be aware of whether the products they use or sell are
likely to be withdrawn, so as to prevent them being left with
stocks of unusable material. Those concerned should contact
their national authority to check the authorisation status for
any particular product. The Regulation (n° 2076/2002 of 20 November
2002), with the list of the 320 substances, has now been published
in the Offical Journa. Ref: MIDDAY EXPRESS. News from the Press
and Communication Service's midday briefing. |
| Glossary
of Pesticide Chemicals, October 2001.
A listing of pesticides subject to analysis of residues in foods
and feeds by the US Food and Drug Administration. |
|
Health
Effects:
As of
Nov 2002: no tox data available |
|
Environmental
Effects
As of
Nov 2002: no tox data available |
PubMed
Abstract:
Protoplasma 2001;216(1-2):80-93
Ultrastructural
effects of cellulose biosynthesis inhibitor herbicides on
developing cotton fibers.
Vaughn
KC, Turley RB.
Southern Weed Science Research Unit, USDA
Agricultural Research Service, P.O. Box 350, Stoneville,
MS 38776, USA.
Cotton fibers are often utilized as a model
system to investigate cellulose biosynthesis and cell wall
elongation. In this study, we grew cotton fibers in vitro,
with ovules dissected at day zero post anthesis as the explant
source, in the presence of three herbicides that inhibit
cellulose biosynthesis. Cultures were sampled for electron
microscopy and immunocytochemistry 1-2 days after beginning
the treatments. After dichlobenil treatment, the fibers
were much shorter than the controls and assumed a variety
of abnormal shapes, from shortened versions of the control
fiber to nearly spherical. The inner layers of the fiber
wall often contained juxtaposed electron-translucent and
-transparent areas; this layer reacted strongly with antibodies
to callose. Cellulase-gold labeling in these newly developed
fibers grown in the presence of dichlobenil was present
at only about 3% of the control labeling. After treatment
with either isoxaben or flupoxam,
the fibers assumed spherical shapes and frequently (more
than 60% of fibers) exhibited a new cell plate within the
fiber, indicating that cell division had occurred, a process
that rarely occurred in the controls. Unlike the dichlobenil-treated
fibers, fibers grown in the presence of isoxaben or flupoxam
contained an extensive accumulation of chiefly deesterified
pectins, replacing the entire wall with an elaborated version
of the pectin sheath found in control cotton fibers. These
data indicate that all three herbicides are effective disrupters
of cellulose biosynthesis and cause radical changes in cell
wall structure and composition. Moreover, these data indicate
that the composition of the walls may influence indirectly
cell cycle kinetics, keeping these fiber cells in a more
meristematic mode.
|
|