|
Return
to Flurprimidol Index Page
Activity: Plant
growth regulator (pyrimidine)
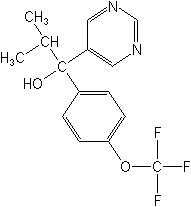
Structure:
Adverse
Effects:
Body
Weight Decrease
Bone
Cholesterol
Endocrine: Adrenal
Endocrine: Ovary (inc. estrous)
Kidney
Liver
Urinary Tract
Environmental
•
No updated toxicological information available since 1989.
However, Eli Lilly & Co. submitted the results of numerous
animal studies to US EPA in 1992, but the results have not
been made public - see
studies.
|
Body
Weight Decrease
(click on for all fluorinated pesticides)
-- A rat teratology
study using doses of 0, 2.5, 10, 45 or 200 mg/kg/day of flurprimidol
had a maternal toxicity NOEL of 10 mg/kg/day and a LEL of 45 mg/kg/day
based on decreased body weight gain and
food consumption. The developmental NOEL was 10
mg/kg/day and the LEL was 45 mg/kg/day based on decreased
fetal weight, increased incidence of hydronephrosis, hydroureter
and numerous developmental
skeletal anomalies.
-- Reproduction Study A 2 generation reproduction study in the
rat treated with (time weighted average) 0, 1.8, 7.3, and 74 mg/kg/day
of flurprimidol had a Parental Systemic Toxicity NOEL of 1.8 mg/kg/day
and a LEL of 7.3 mg/kg/day based on increased incidence of non-neoplastic
hepatocellular alterations including fatty change and vacuolation
(males) and increased susceptibility to stress factors. The Reproductive
NOEL was 7.3 mg/kg/ and the LEL was 74 mg/kg/day based on decreased
mating, fertility, fetal survival (stillbirths), neonatal survival
and neonatal body weight in both sexes and
in both generations. There was an increased incidence of
persistent vaginal estrous and no corpora lutea. Additional parental
signs of toxicity at 74 mg/kg/day included increased susceptibility
to stress (pregnant females) resulting in death, increased relative
liver weight (males and females), depressed
body weight, weight gain and food consumption (males and
females).
Ref: US EPA Pesticide Fact Sheet for
Flurprimidol. February 22, 1989.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Bone
(click on for all fluorinated
pesticides)
A rat teratology study
using doses of 0, 2.5, 10, 45 or 200 mg/kg/day of flurprimidol
had a maternal toxicity NOEL of 10 mg/kg/day and a LEL of 45 mg/kg/day
based on decreased body weight gain and food consumption. The
developmental NOEL was 10 mg/kg/day and the LEL was 45 mg/kg/day
based on decreased fetal weight, increased incidence of hydronephrosis,
hydroureter and numerous developmental skeletal
anomalies.
Ref: US EPA Pesticide Fact Sheet.
February 22, 1989.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Cholesterol
(click
on for all fluorinated pesticides)
Chronic Studies. Rodent
Feeding Studies. A 2-year study in rats treated with either 0,
1.0, 3.6, 12.1 and 41.2 mg/kg/day of flurprimidol technical for
males and 0, 1.2, 4.4, 14.5, and 49.3 mg/kg/day for females the
NOEL was 3.6 mg/kg/day and the LEL was 12.1 mg/kg/day based upon
hepatocellular changes in males including enzyme induction, fatty
change, hepatocellular eosinophilic change and focal atypia. At
41.2 mg/kg/day there was also a transient body weight and weight
gain decrease (males), increased cholesterol
and triglycerides (males and females) increased hepatic enzyme
induction and liver weight, fatty change and hepatocellular eosinophilic
change (females). No oncogenic potential was observed at any dose
level.
Ref: EPA Pesticide Fact Sheet Name: Flurprimidol.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Endocrine:
Adrenal (click
on for all fluorinated pesticides)
-- A l-year dog study
treated with flurprimidol at doses of 0, 0.5, 1.5, 7.0 and 30.0
mg/kg/day, the NOEl was 7.0 mg/kg/day and the LEL was 30.0 mg/kg/day
Highest Dose Tested (HDT) based on adrenal
changes including decreased plasma cortisol response to ACTH stimulation
(males and degenerative changes of the adrenal
cortex (males and females). The histopathology was limited
to the zona fasciculata of the adrenal cortex
and was characterized by eosinophilic degeneration, vacuolation
and cortical atrophy. There was a slight increase in hepatic p-nitroanisole
0-demethylase activity (males).
Ref: EPA Pesticide Fact Sheet for Flurprimidol.
Reason for Issuance: New Chemical Registration. Date Issued: FEB
22 1989. Fact Sheet Number: 202.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Endocrine:
Ovary (click
on for all fluorinated pesticides)
-- Subchronic Studies.
A 90-day feeding study in rats treated to 0, 1.68, 6.04, 20.39,
68.34 mg/kg/day (males) and 0, 1.98, 7.13, 24.37, 78.47 mg/kg/day
(females) of flurprimidol. The systemic No-Observable- Effect-Level
(NOEL) was 1.68 mg/kg/day and the Lowest-Effect- Level (LEL) was
6.04 mg/kg/day based on increased hepatic
enzyme induction in males (significant and dose increases
in p- nitroanisol O-demthylase activity). At 24.37 mg/kg/day there
was increased relative and absolute ovarian
(female) and relative liver (male) weight.
At 68.34 mg/kg/day, there was increased absolute liver weight
(males).
Ref: EPA Pesticide Fact Sheet for Flurprimidol.
Reason for Issuance: New Chemical Registration. Date Issued: FEB
22 1989. Fact Sheet Number: 202.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
-- Reproduction Study
A 2 generation reproduction study in the rat treated with (time
weighted average) 0, 1.8, 7.3, and 74 mg/kg/day of flurprimidol
had a Parental Systemic Toxicity NOEL of 1.8 mg/kg/day and a LEL
of 7.3 mg/kg/day based on increased incidence of non-neoplastic
hepatocellular alterations including fatty change and vacuolation
(males) and increased susceptibility to stress factors. The Reproductive
NOEL was 7.3 mg/kg/ and the LEL was 74 mg/kg/day based on decreased
mating, fertility, fetal survival (stillbirths), neonatal survival
and neonatal body weight in both sexes and in both generations.
There was an increased incidence of persistent vaginal
estrous and no corpora lutea.
Additional parental signs of toxicity at 74 mg/kg/day included
increased susceptibility to stress (pregnant females) resulting
in death, increased relative liver weight (males and females),
depressed body weight, weight gain and food consumption (males
and females)...
Ref: EPA Pesticide Fact Sheet for Flurprimidol.
Reason for Issuance: New Chemical Registration. Date Issued: FEB
22 1989. Fact Sheet Number: 202.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Kidney
(click on for all fluorinated
pesticides)
A rat teratology study
using doses of 0, 2.5, 10, 45 or 200 mg/kg/day of flurprimidol
had a maternal toxicity NOEL of 10 mg/kg/day and a LEL of 45 mg/kg/day
based on decreased body weight gain and
food consumption. The developmental NOEL was 10 mg/kg/day and
the LEL was 45 mg/kg/day based on decreased fetal weight, increased
incidence of hydronephrosis,
hydroureter and
numerous developmental skeletal
anomalies.
Ref: US EPA Pesticide Fact Sheet. February
22, 1989.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
•
Hydronephrosis:
Pathological
chronic enlargement of the collecting channels of a kidney, leading
to compression and eventual destruction of kidney tissue, and
diminishing kidney functionning.
http://www.hyperdictionary.com/medical/hydronephrosis
Liver
(click on for all fluorinated
pesticides)
Summary Science Statement:
-- Chronic feeding/oncogenicity studies were conducted in both
the rat and mouse. Hepatocellular changes
in the males including enzyme induction, fatty change, hepatocellular
eosinophilic change and focal atypia were observed in the
rat study. A core- supplementary mouse study showed increased
absolute and relative liver weight
in females. Although both the rat and mouse study are core-supplementary
for oncogenicity due to inadequate dose selection, they both satisfy
the requirement for oncogenicity testing in one species for the
requested non-food uses. No oncogenic potential was observed at
any dose level in either of these two studies. New rat and mouse
studies (which achieve the Maximum Tolerated Dose - MTD) will
be required for any food use registrations.
-- The requirements for a 90-day feeding study have been satisfied.
A subchronic oral rat study showed an increased hepatic enzyme
induction in males (significant and dose increases in p-nitroanisol
o-demethylase activity). The subchronic oral mouse study indicated
an increased incidence of hepatocellular
hypertrophy in the males.
-- The requirements for a 2 generation reproductive study have
been satisfied. The Parental Systemic Toxicity in a 2 generation
reproduction study showed increased incidence of non-neoplastic
hepatocellular alteration including
fatty change and vacuolation (males) and increased susceptibility
to stress factors. Decreased mating, fertility, fetal survival
(stillbirths), neonatal survival and neonatal body weight in both
sexes and in both generations were observed at the Reproductive
NOEL. other parental signs of toxicity included increased susceptibility
to stress (pregnant females) resulting in death, increased relative
liver weight (males and females), depressed body weight,
weight gain and food consumption (males and females).
-- Subchronic Studies. A 90-day feeding study in rats treated
to 0, 1.68, 6.04, 20.39, 68.34 mg/kg/day (males) and 0, 1.98,
7.13, 24.37, 78.47 mg/kg/day (females) of flurprimidol. The systemic
No-Observable- Effect-Level (NOEL) was 1.68 mg/kg/day and the
Lowest-Effect- Level (LEL) was 6.04 mg/kg/day based on increased
hepatic enzyme induction in males
(significant and dose increases in p- nitroanisol O-demthylase
activity). At 24.37 mg/kg/day there was increased relative and
absolute ovarian (female) and relative
liver (male) weight. At 68.34 mg/kg/day,
there was increased absolute liver weight
(males).
-- A 90-day feeding study in the mouse, treated with 0, 15, 67.5
and 300 mg/kg/day of technical flurprimidol. The NOEL was 15 mg/kg/day
and the LEL was 67.5 mg/kg/day based on increased incidence of
hepatocellular hypertrophy in the males.
At 300 mg/kg/day, there was evidence of enzyme induction, increased
liver weight and
hepatocellular hypertrophy in females.
-- Chronic Studies - Rodent Feeding Studies.
A 2-year study in rats treated with either 0, 1.0, 3.6, 12.1 and
41.2 mg/kg/day of flurprimidol technical for males and 0, 1.2,
4.4, 14.5, and 49.3 mg/kg/day for females the NOEL was 3.6 mg/kg/day
and the LEL was 12.1 mg/kg/day based upon hepatocellular
changes in males including enzyme induction, fatty change,
hepatocellular eosinophilic change
and focal atypia. At 41.2 mg/kg/day there was also a transient
body weight and weight gain decrease (males), increased cholesterol
and triglycerides (males and females) increased hepatic enzyme
induction and liver weight, fatty
change and hepatocellular eosinophilic change
(females). No oncogenic potential was observed at any dose
level.
-- A 2-year core-supplementary study in mice treated with either
0, 1.4, 10.5 or 79.9 mg/kg/day of flurprimidol, the systemic NOEl
was 1.4 mg/kg/day. The LEL was 10.5 mg/kg/day based on increased
absolute and relative liver weight in the
males. No oncogenic potential was observed at any dose
level.
-- Reproduction Study, A 2 generation reproduction study in the
rat treated with (time weighted average) 0, 1.8, 7.3, and 74 mg/kg/day
of flurprimidol had a Parental Systemic Toxicity NOEL of 1.8 mg/kg/day
and a LEL of 7.3 mg/kg/day based on increased incidence of non-neoplastic
hepatocellular alterations including
fatty change and vacuolation (males) and increased susceptibility
to stress factors. The Reproductive NOEL was 7.3 mg/kg/ and the
LEL was 74 mg/kg/day based on decreased mating, fertility, fetal
survival (stillbirths), neonatal survival and neonatal body weight
in both sexes and in both generations. There was an increased
incidence of persistent vaginal estrous
and no corpora lutea. Additional parental signs of toxicity
at 74 mg/kg/day included increased susceptibility to stress (pregnant
females) resulting in death, increased relative liver
weight (males and females), depressed body weight, weight
gain and food consumption (males and females).
Ref: EPA Pesticide Fact Sheet for Flurprimidol.
Reason for Issuance: New Chemical Registration. Date Issued: FEB
22 1989. Fact Sheet Number: 202.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
Urinary
Tract
(click on for all fluorinated
pesticides)
A rat
teratology study using doses of 0, 2.5, 10, 45 or 200 mg/kg/day
of flurprimidol had a maternal toxicity NOEL of 10 mg/kg/day and
a LEL of 45 mg/kg/day based on decreased
body weight gain and food consumption. The developmental NOEL
was 10 mg/kg/day and the LEL was 45 mg/kg/day based on decreased
fetal weight, increased incidence of hydronephrosis, hydroureter
and numerous developmental
skeletal anomalies.
Ref: US EPA Pesticide Fact Sheet. February
22, 1989.
http://www.fluoridealert.org/pesticides/Flurprimidol.EPA.Facts.1989.htm
|

