|
Return to Fuazifop-butyl
Index Page
Return to Abstracts
Activity: Herbicide
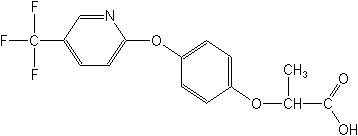
Structure:
Adverse
Effects:
Blood
Body
Weight Decrease
Bone
Brain
Cholesterol
Endocrine:
Adrenal
Endocrine:
Ovary
Endocrine: Pituitary
Endocrine: Suspected
Endocrine Disruptor
Endocrine: Testicular
Endocrine: Thymus
Endocrine:
Uterus
Eye
Fetotoxic
Gastrointestinal Tract
Kidney
Liver
Mesenteric Lymph Nodes
Reproductive
Spleen
Teratogenic
Environmental
Rationale
for US EPA to add Fluazifop butyl
to the Toxic Release Inventory
A 3-month
rat feeding study demonstrated hepatocyte hypertrophy in
males (the LOEL was 5 mg/kg/day; the NOEL was 0.5 mg/kg/
day). In a 1-year feeding study, dogs had changes in serum
alkaline phosphatase and alanine aminotransferase and/or
alanine sulfatransferase (the LOEL was 25 mg/kg/day; the
NOEL was 5 mg/kg/day). Similar changes were also reported
in dogs following 3 months exposure in their diet (the LOEL
was 125 mg/kg/day). In a carcinogenicity study, male mice
fed 20 ppm (2.6 mg/kg/day, the LOEL) had an increased incidence
of hepatocyte hypertrophy. The NOEL was 5 ppm or 0.65 mg/kg/
day. Male and female mice exposed to a higher dose of 80
ppm (10.4 mg/ kg/day) had increased liver weight (relative
and absolute) and hypertrophy of periacinal hepatocytes.
Males in this dose group also had increased pigmentation
in hepatocytes and Kupffer cells. In a teratogenicity study
in Sprague-Dawley rats exposed via oral gavage, delayed
ossification and an increased incidence of hydroureter were
observed in fetuses (the fetotoxic LOEL was 5 mg/kg/day;
the NOEL 1 mg/kg/day) and a teratogenic LOEL of 200 mg/kg/day
(the NOEL was 10 mg/kg/day) was determined based on the
incidence of diaphragmatic hernia. Maternal toxicity was
observed in this study at doses higher than those causing
fetotoxicity and included reduced body weight gain and decreased
gravid uterus (the maternal LOEL was 200 mg/kg/day; the
NOEL was 10 mg/kg/day). In a 2-generation reproductive toxicity
dietary study in Wistar rats, the reproductive LOEL of 250
ppm (12.5 mg/kg/day; the NOEL was 80 ppm or 4 mg/kg/day)
was based on reduced litter sizes, reduced viability, reduced
testis and epididymis weights and tubular atrophy in offspring.
Fetotoxicity (delayed ossification and eye opacities) was
also demonstrated in New Zealand White rabbits (the LOEL
was 30 mg/kg/day; the NOEL was 10 mg/kg/day). EPA believes
that there is sufficient evidence for listing fluazifop
butyl on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B)
based on the available hepatic and developmental toxicity
data for this chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals
from Federal Insecticide, Fungicide, Rodenticide Act (FIFRA)
Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993).
As cited by US EPA in:
Federal
Register: January 12, 1994. Part
IV. 40 CFR Part 372. Addition of Certain Chemicals; Toxic
Chemical Release Reporting; Community Right-to-Know; Proposed
Rule.
|
Blood
(click on for all fluorinated pesticides)
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study.
Fluazifop-butyl caused ulcerations in the g.i. tract, specifically
in the tongue, lip, mouth lining, stomach pylorus, or cecum. Ulcerations
may have contributed to low RBC parameters in both sexes (reduced
HCT, Hb levels, and RBC counts, particularly in males). Platelet
counts were reduced by about half in both sexes.
Bone
marrow samples
taken at weeks 10 and 52 for smear analyses showed "reduced
numbers of megakaryocytes," suggesting reduced production
as a reason for low platelet counts. Other
hematology-related findings included increased incidence/degree
of thymic involution, decreased lymphocyte counts, increased degree
of hemosiderin-laden Kupffer cells, hypercellular
sternal marrow, and severe extramedullary hematopoiesis in 4 male
and 1 female decedent. Four of the latter 5 dogs
also had substantially increased splenic
weights, "pallor" in clinical signs as part of
moribund condition, and their final hematology
red cell values (RBC count, Hb, HCT) were very low.
Eyes of eight high dose dogs had cataracts, usually accompanied
by miliary ("seed-like" appearance) vacuolation of the
lens. Liver dysfunction was indicated by periacinar hepatocytic
degeneration and thinning of hepatic cords in some dogs, other
hepatocytic changes such as vacuolation and/or granular cytoplasm,
and occasional bile plugs in the canaliculi. Most clinical chemistry
changes were plausibly related to liver toxicity, including
elevated alkaline phosphatase, ALT, and occasionally AST.
Substantially increased BSP retention was consistent with biliary
disturbance. Urine was typically bright yellow or orange due to
high bile pigment concentrations. Cholesterol
was consistently reduced. Male reproductive toxicity included
testicular tubular degeneration and reduced/absent
spermatozoa in epididymides. Possible adverse effects (many-faceted
toxicity, including mortalities, at 125 mg/kg/day). Acceptable.
Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- Fluazifop butyl
(CAS # 69806-50-4) was evaluated for developmental toxicity in
the progeny of Sprague-Dawley CD rats administered
oral doses of 0 (corn oil vehicle control), 10, 50 and 200 mg/kg/day
by gavage on gestation days 6-20... Delayed fetal ossification
and reduced fetal weight were dose-related
and evident at all treatment levels, although fetal weights
in association with dosages of 10 and 50 mg/kg/day were within
the threshold of historical controls... [ICI AMERS INC; Butyl
2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy) propionate butyl:
Effects of Oral Administration Upon Pregnancy in Rats; 05/23/80;
EPA Doc No. 88-920006839; Fiche No. OTS0543844]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
In a teratogenicity
study in Sprague-Dawley rats exposed via oral gavage, delayed
ossification and an increased incidence of hydroureter were observed
in fetuses (the fetotoxic LOEL was 5 mg/kg/day; the NOEL 1 mg/kg/day)
and a teratogenic LOEL of 200 mg/kg/day (the NOEL was 10 mg/kg/day)
was determined based on the incidence of diaphragmatic hernia.
Maternal toxicity was observed in this study at doses higher than
those causing fetotoxicity and included reduced
body weight gain and decreased gravid uterus (the maternal
LOEL was 200 mg/kg/day; the NOEL was 10 mg/kg/day). In a 2-generation
reproductive toxicity dietary study in Wistar rats, the reproductive
LOEL of 250 ppm (12.5 mg/kg/day; the NOEL was 80 ppm or 4 mg/kg/day)
was based on reduced litter sizes,
reduced viability, reduced testis and epididymis weights and tubular
atrophy in offspring. Fetotoxicity (delayed ossification and eye
opacities) was also demonstrated in New Zealand White rabbits
(the LOEL was 30 mg/kg/day; the NOEL was 10 mg/kg/day). EPA believes
that there is sufficient evidence for listing fluazifop butyl
on EPCRA section 313 pursuant to EPCRA section 313(d)(2)(B) based
on the available hepatic and developmental toxicity data for this
chemical.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993). As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
Fluazifop
butyl
was evaluated for developmental toxicity in adult virgin Sprague-Dawley
CD rats (160/treatment group) administered oral doses of 0, 1,
5, 10 and 200 mg/kg/day during gestation days 6-20. No increased
maternal mortality or overt toxicity was attributed to treatment.
At gestation Day 21 sacrifice of the dams, a
slight but significantly depressed mean bodyweight gain among
those of a 200 mg/kg/day dosage was indicative of dose-
related and significant (p < 0.05; Student's t-test) reduction
in gravid uterus weights. Significant (p < 0.01;
Student's t-tests) dosage-related reductions
in fetal weights in association with treatment from 5 mg/kg/day
were found to correlate with increased incidence of small
fetuses; Placental weights were also
significantly low in association with treatment at 200 mg/kg/day
relative to controols. Gross pathological changes included increased
incidence of diaphragmatic hernia (4.4% in association with 200
mg/kg/day) noted at all treatment levels relative to controls
(0%), and slight dosage-related increased occurrence of hydroureter,
partnered with a marginal increase in hydronephrosis in 200 mg/kg/day
fetuses. Micropathological investigation validated dosage-related
incidence of hydroureter, hydronephrosis in association with a
200 mg/kg/day regimen, and subcutaneous edema among 200 mg/kg/day
fetuses. Skeletal examinations further revealed retarded ossification
that was dosage-related in fetuses of gestational treatment levels
at 5 mg/kg/day and above. Treatment levels of 1 mg/kg/day
were associated with no toxic, reproductive or developmental consequences
relative to controls under the conditions of this study.
Ref: 1992
- INITIAL SUBMISSION: TERATOLOGY STUDY WITH FLUAZIFOP BUTYL IN
RATS WITH COVER LETTER DATED 08-28-92. LIFE SCI RESEARCH. NTIS/OTS0545395
and EPA/OTS; Doc #88-920007020. [Abstract from Toxline at Toxnet.]
Bone
(click on for all fluorinated pesticides)
-- Fluazifop butyl
(CAS # 69806-50-4) was evaluated for developmental toxicity in
the progeny of Sprague-Dawley CD rats administered oral doses
of 0 (corn oil vehicle control), 10, 50 and 200 mg/kg/day by gavage
on gestation days 6-20... Delayed fetal
ossification and reduced fetal weight were dose-related
and evident at all treatment levels, although fetal weights in
association with dosages of 10 and 50 mg/kg/day were within the
threshold of historical controls... [ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate butyl: Effects of Oral Administration Upon Pregnancy
in Rats; 05/23/80; EPA Doc No. 88-920006839; Fiche No. OTS0543844]
-- Fluazifop butyl was evaluated for developmental toxicity in
adult virgin Sprague-Dawley CD rats (160/group) administered oral
doses of 0, 1, 5, 10 and 200 mg/kg/day during gestation days 6-20...
Skeletal examinations further revealed retarded
ossification that was dosage-related in fetuses of gestational
treatment levels of 5 mg/kg/day and above... [ICI AMERS INC; Teratology
Study with Fluazifop Butyl in Rats; 06/03/81; EPA Doc No. 88-920007020;
Fiche No. OTS0545395]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study...
Bone
marrow samples
taken at weeks 10 and 52 for smear analyses showed "reduced
numbers of megakaryocytes •,"
suggesting reduced production as a reason for low platelet counts.
Other hematology-related findings included
increased incidence/degree of thymic involution, decreased lymphocyte
counts, increased degree of hemosiderin-laden Kupffer cells,
hypercellular sternal marrow, and severe
extramedullary hematopoiesis ••
in 4 male and 1 female decedent....
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
•
Megakaryocytes - Very large bone marrow cells which
release mature blood platelets.
••
Hematopoiesis
- The formation and development of blood cells, usually takes
place in the bone marrow.
4.2.3.2 Fluazifop-butyl - Sprague Dawley Rats In a developmental
toxicity study (MRID# 00088857, 92067047 and 92067019), fluazifop-butyl,
[PP009 (94.8% a.i., batch/lot # P14)] was administered to 22 female
CD Sprague Dawley strain rats/group in a corn oil (2 ml/kg) gavage
at dose levels of 0, 10, 50 or 200 mg/kg bw/day from days 6 through
20 of gestation. Animals were sacrificed on day 21 ... Various
parameters significant to development were affected relative to
concurrent and/or historical controls. Post
implantation loss was increased (125%) at 200 mg/kg/day.
Examination of the heads of fetuses showed
increased large fontanelles at 200 and 50 mg/kg/day (45.9%
and 11.9%, respectively, versus 3.4% in concurrent controls.
Increased incomplete and/or irregular ossification of cranial
sutures at 200 and 50 mg/kg/day (58.9% and 43.7%, respectively,
versus 14.3% in concurrent controls. The incidence of fissures
into the interparietal bone was increased at 200 mg/kg/day (2.2%
versus 0% in concurrent and historical controls. Only the
percentages of fetal anomalies were presented with no litter incidence.
No statistical analysis was presented for the fetal anomalies.
Increased incidence of incomplete ossification of thoracic vertebral
centra at 200, 50 and 10 mg/kg/day (75.`5%, 58.7, 48.4, respectively,
versus 38.5% in concurrent controls, with a historical control
range of 0-70.3%) appeared to be test material related.
An increased incidence of absent hyoid bone
at 200 and 50 mg/kg/day (23.0% and 17.2% versus 10.8% in concurrent
controls) was observed. Increased incidence of incomplete ossification
of one or more pelvic bones at 200 and 50 mg/kg/day (7.4% and
2.3%, respectively, versus 1.4% in concurrent control) was also
observed. Absent hyoid bone and incomplete and/or irregular
ossification of the cranial bones, and incomplete ossification
of one or more pelvic bones may have been increased at 50 mg/kg/day,
but since concurrent controls were higher than the mean historical
control, these effects may have been incidental. The
incidence of bilateral hydronephrosis at 200 mg/kg/day exceeded
the historical control mean but not the range. The
incidence of bilateral hydroureter at 200 mg/kg/day exceeded the
historical control range and mean. No hydronephrosis nor
hydroureter were seen in concurrent controls. In addition, subcutaneous
edema (17.7% at 200 mg/kg/day versus 3.4% in concurrent controls)
exceeded the mean of 8.9% in historical controls (the upper range
was unreadable). The concurrent controls also were less
than the mean of historical controls. Diaphragmatic hernia was
seen in 1 fetus at 10 mg/kg/day, none at 50 mg/kg/day, and in
3 fetuses at 200 mg/kg/day, with no incidences in 2970 historical
control fetuses. However a subsequent much
larger study (MRID# 00088858) with 160 litter/group, showed an
incidence of diaphragmatic hernias of 3/1113 in control fetuses,
1/1081 fetuses at 1 mg/kg/day, 3/1073 fetuses at 5 mg/kg/day,
2/1064 fetuses at 10 mg/kg/day, and a statistically significantly
increased incidence in 59/1064 fetuses and 45/159 litters at 200
mg/kg/day. Since this anomaly was seen at a higher incidence
(3 fetuses) in the controls in this study and was not replicated
at the same dose in the second study, the single incidence of
diaphragmatic hernia at 10 mg/kg/day was considered to be an aberration
and not attributable to treatment. For developmental
toxicity, the LOAEL is 10 mg/kg/day based on incomplete and/or
irregular ossification of the cranial bones and incomplete ossification
of thoracic vertebral centra; a NOAEL was not established.
Ref:
December 10, 2004. US
EPA> Fluazifop-P-butyl: Revised HED Chapter of the Tolerance
Reassessment Eligibility Decision (TRED) Document. EPA Docket
number: OPP-2004-0347-0003
http://www.fluorideaction.org/pesticides/f-p-b.opp-2004-0347-0003.pdf
4.2.3.4 Fluazifop-P-butyl - Wistar Rats In a developmental toxicity
study (MRID 46028903) [Fluazifop-p-butyl, calculated as 90.9%
a.i.; batch/lot# BX 247T A10209)] was administered to [(24 females)
Alderly Park strain of Wistar rats] rats/dose by gavage at dose
levels of 0, 2, 5 or 100 mg a.i./kg bw/day from days 7 through
21 of gestation ... Delayed ossification
was seen in skull bones. The incidence of the partially ossified
occipital (3.3% to 7.1% versus 0% in control), interparietals
(9.5% to 29.5% versus 0.4% in control, historical controls), and
in parietal bones (14.2% to 38.2% versus 0.4% in control) were
dose related and statistically significant in fetuses and litters
at 5.0 mg/kg/day and above. [In this study, historical
control data are useful but limited because it was collected on
studies with dosing gestational day 7 - 16.] The mean manus [10%
to 30% of control] and pes scores [4% to 14% of control] showed
a statistically significant dose related increase delayed ossification,
starting at 5 mg/kg/day. [Manus and pes scores (1-6) were
a subjective index of delayed ossification (1-6) in the fore paws
and hind paws of each fetus.] Other indications
of delayed ossification were seen at the highest dose tested for
cervical vertebral arches and centrum (not ossified) and sternebrae
5 and 6. In addition, the odontoid [tooth related] and calcaneum
[heel bone] were not ossified at 100 mg/kg/day. Fetal weight
was significantly decreased 7% at 100 mg/kg/day only. Increased
incidence of dilated ureter was seen only in litters at 100 mg/kg/day
and kinked ureter in was dose related and statistically significant
at all doses in fetuses, but not in litters at any dose level.
There were no incidences of diaphragmatic hernias seen in this
study. For developmental toxicity, the NOAEL is 2.0 mg/kg/day
and the LOAEL is 5.0 mg/kg/day based on dose related delayed ossification
in skull bones [occipital and parietal] in fetuses and litters
(page 34-35).
Ref:
December 10, 2004. US
EPA> Fluazifop-P-butyl: Revised HED Chapter of the Tolerance
Reassessment Eligibility Decision (TRED) Document. EPA Docket
number: OPP-2004-0347-0003
http://www.fluorideaction.org/pesticides/f-p-b.opp-2004-0347-0003.pdf
Brain
(click on for all fluorinated pesticides)
Fluazifop butyl was
evaluated for reproductive and developmental effects in 2 successive
generations of Charles River Wistar strain rats (30/sex/group)
exposed continuously to 0, 10, 80 and 250 ppm in the diet. Each
respective parent generation had received treatment for a minimum
of 100 days (F0) and 120 days (F1) prior to mating. F0 and F1
dams weaned their progeny for 25 days postpartum to time of offspring
selection for mating and continued study (F1) or sacrifice (F1,
F2). Thirty days after sacrifice of their offspring, the surviving
F1 females and select F1 males were sacrificed and with representative
F1 and F2 offspring were examined histologically... .
Testis and epididymis weights in the males (80, 250 ppm), and
pituitary gland (80, 250 ppm), uterus (80, 250 ppm),
brain (250 ppm) and lung weights (250 ppm)
in females were significantly reduced...
[ICI AMERS INC; Fluazifop butyl:
Effects Upon Reproductive Performance of Rats Treated Continuously
Through 2 Generations (Final Report); 03/17/81; EPA Doc No. 88-920006849;
Fiche No. OTS05543854]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
Cholesterol
(click on for all fluorinated pesticides)
-- Fluazifop-butyl
(CAS # 69806-50-4) was evaluated for subchronic dietary toxicity
in Wistar rats (20/sex/group) receiving 0, 10, 100 and 2000 ppm
by dietary inclusion for 13 weeks. Mean achieved dosages during
13-week treatment were 0, 0.7, 7.1 and 144.5 mg/kg/day in males
of the respective treatment groups and 0, 0.8, 8.0 and 161.9 mg/kg/day
in females... cholesterol and total
protein concentrations were low as compared to controls. [ICI
AMERICAS INC; 13 Week Dietary Toxicity Study with PP009 in Rats
(Final Report); 05/29/80; EPA Doc No. 88-920006943; Fiche No.
OTS0545346]
-- Fluazifop-butyl (CAS # 69806-50-4) was evaluated for subacute
oral toxicity in Wistar albino rats (10/sex/group) administered
10 ml/kg doses in corn oil of 0, 4, 20, 100 and 500 mg/kg/day
by oral gavage, 5 days/week for 2 weeks. Five-day weekly treatment
periods were each followed by 2-day recovery... Blood chemistry
indicated a dose-related elevation of alkaline phosphatase activity
in 100 and 500 mg/kg/day males, elevated aspartate amino-transferase
activity in 500 mg/kg/day males, and elevated
cholesterol concentrations in 500 mg/kg/day males and females.
Conversely, enzyme activity was depressed in rats of lower dosages...
[ICI AMERS INC; 14 Day Subacute Oral Toxicity Study with PP009
in Rats; 07/11/80; EPA Doc No. 88-920007017; Fiche No. OTS0545392]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study
... Cholesterol
was consistently reduced. Male reproductive
toxicity included testicular tubular degeneration and reduced/absent
spermatozoa in epididymides. Possible adverse effects
(many-faceted toxicity, including mortalities, at 125 mg/kg/day).
Acceptable. Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Endocrine:
Adrenal
(click on for all fluorinated pesticides)
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day. The degree of
adrenal gland cortical fatty degeneration evident in one
mid-dose female was sufficiently greater than that of any of the
control dogs that investigators attributed this finding to treatment.
Cortical fatty degeneration of moderate
to severe degree was observed in one high dose male and in all
high dose females. All other noteworthy findings were limited
to the high dose group, as follows...
Male reproductive toxicity included testicular tubular degeneration
and reduced/absent spermatozoa in epididymides.
Possible adverse effects (many-faceted toxicity, including
mortalities, at 125 mg/kg/day). Acceptable. Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Endocrine:
Ovary
(click on for all fluorinated
pesticides)
--
Fluazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2). Thirty days after sacrifice of their
offspring, the surviving F1 females and select F1 males were sacrificed
and with representative F1 and F2 offspring were examined histologically...
Necropsy of F0 parents revealed no gross pathology and, although
testes and epididymis of F0 males of a 250 ppm exposure were reduced,
histological review identified no treatment-related changes. Conversely,
F1 parents were found with gross indications of toxicity.. . Testis
and epididymis weights in the males (80, 250 ppm), and pituitary
gland (80, 250 ppm), uterus (80, 250 ppm), brain (250 ppm) and
lung weights (250 ppm) in females were significantly reduced.
Female F1 ovarian weights
were increased relative to controls in association with a 250
ppm dietary exposure. Upon histological investigation, increased
incidence geriatric nephropathy [kidney](both sexes, 80 and 250
ppm), distension of mesenteric and/or cervical
lymph nodes (250 ppm) and increased severity of nephrocalcinosis
(females, 80 and 250 ppm), and an increased slight testicular
tubular atrophy in males (250 ppm) were noted... [ICI
AMERS INC; Fluazifop butyl: Effects Upon Reproductive Performance
of Rats Treated Continuously Through 2 Generations (Final Report);
03/17/81; EPA Doc No. 88-920006849; Fiche No. OTS05543854]
Ref:
TOXNET profile from Hazardous Substances Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
Endocrine:
Pituitary
(click on for all fluorinated pesticides)
--
Fluazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2). Thirty days after sacrifice of their
offspring, the surviving F1 females and select F1 males were sacrificed
and with representative F1 and F2 offspring were examined histologically...
Necropsy of F0 parents revealed no gross pathology and, although
testes and epididymis of F0 males of a 250 ppm exposure were reduced,
histological review identified no treatment-related changes. Conversely,
F1 parents were found with gross indications of toxicity.. . Testis
and epididymis weights in the males (80, 250 ppm), and
pituitary gland (80, 250 ppm), uterus
(80, 250 ppm), brain (250 ppm) and lung weights (250 ppm) in females
were significantly reduced. Female
F1 ovarian weights were increased relative to controls in association
with a 250 ppm dietary exposure. Upon histological investigation,
increased incidence geriatric nephropathy [kidney](both sexes,
80 and 250 ppm), distension of mesenteric and/or cervical lymph
nodes (250 ppm) and increased severity of nephrocalcinosis (females,
80 and 250 ppm), and an increased slight testicular tubular atrophy
in males (250 ppm) were noted... [ICI
AMERS INC; Fluazifop butyl: Effects Upon Reproductive Performance
of Rats Treated Continuously Through 2 Generations (Final Report);
03/17/81; EPA Doc No. 88-920006849; Fiche No. OTS05543854]
Ref:
TOXNET profile from Hazardous Substances Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
Endocrine:
Suspected Endocrine Disruptor
(click on for all fluorinated pesticides)
Suspected
Endocrine Disruptor
Ref: June 14, 2001 - Implementation of the
Community Strategy for Endocrine Disruptors - a range of substances
suspected of interfering with the hormone systems of humans and
wildlife. Communication from the Commission to the Council and
the European Parliament. Commission of the European Communities,
Brussels COM (2001) 262 final.
http://www.fluoridealert.org/pesticides/Endocrine.Disruptors.EC2001.pdf
More information available at:
http://europa.eu.int/eur-lex/en/com/cnc/2001/com2001_0262en01.pdf
Endocrine:
Testicular
(click on for all fluorinated pesticides)
--
Fluazifop-butyl (CAS # 69806-50-4) was evaluated for subchronic
oral toxicity in beagle dogs (4/sex/group) administered doses
of 0 (vehicle control, corn oil), 5, 25 and 250 mg/kg/day in gelatin
capsules for 13 weeks. Severe corneal ulceration, requiring humane
sacrifice of 2 males and 1 female of a 250 mg/kg/day dosage during
Week 4, prompted adjustment of this regimen to 125 mg/kg/day for
the remainder of study... Histopathological evaluation confirmed
treatment-related lesions of the eyes, liver,
testes and gastrointestinal tract... Conversely, all dogs
surviving 13-week treatment showed no clinical signs of toxicity,
and no treatment-related changes on physical and ophthalmic examinations
at Weeks 4, 8 and 12. .. Upon terminal necropsy, organ weights
and gross pathology of dogs surviving 13-week study were unremarkable
and histopathological analysis identified a solitary incidence
of arrested maturation in the testicular
germinal epithelium of one high-dose male.
[ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate: 13-Week Oral Toxicity Study in Beagle Dogs; 04/24/80;
EPA Doc No. 88-920006846; Fiche No. OTS0543851]
-- Fluazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2). Thirty days after sacrifice of their
offspring, the surviving F1 females and select F1 males were sacrificed
and with representative F1 and F2 offspring were examined histologically...
Necropsy of F0 parents revealed no gross pathology and, although
testes and epididymis
of F0 males of a 250 ppm exposure were reduced, histological review
identified no treatment-related changes. Conversely, F1 parents
were found with gross indications of toxicity.. . Testis
and epididymis weights in the males (80, 250 ppm), and
pituitary gland (80, 250 ppm), uterus (80, 250 ppm), brain (250
ppm) and lung weights (250 ppm) in females were significantly
reduced. Female F1 ovarian
weights were increased relative to controls in association with
a 250 ppm dietary exposure. Upon histological investigation, increased
incidence geriatric nephropathy [kidney](both sexes, 80 and 250
ppm), distension of mesenteric and/or cervical lymph nodes
(250 ppm) and increased severity of nephrocalcinosis (females,
80 and 250 ppm), and an increased slight testicular
tubular atrophy in males (250 ppm) were noted... [ICI
AMERS INC; Fluazifop butyl: Effects Upon Reproductive Performance
of Rats Treated Continuously Through 2 Generations (Final Report);
03/17/81; EPA Doc No. 88-920006849; Fiche No. OTS05543854]
Ref:
TOXNET profile from Hazardous Substances Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
-- 411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... Male reproductive
toxicity included testicular tubular degeneration and reduced/absent
spermatozoa in epididymides. Possible adverse effects (many-faceted
toxicity, including mortalities, at 125 mg/kg/day). Acceptable.
Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Endocrine:
Thymus
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study...
Platelet counts were reduced by about half
in both sexes. Bone
marrow samples taken at weeks 10 and 52 for smear analyses showed
"reduced numbers of megakaryocytes," suggesting reduced
production as a reason for low platelet counts. Other hematology-related
findings included
increased incidence/degree of thymic involution,
decreased lymphocyte counts, increased degree of hemosiderin-laden
Kupffer cells, hypercellular sternal marrow, and severe extramedullary
hematopoiesis in 4 male and 1 female decedent.
Four of the latter 5 dogs also had substantially increased splenic
weights, "pallor" in clinical signs as part of moribund
condition, and their final hematology red cell values (RBC count,
Hb, HCT) were very low....
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
• Thymic involution:
-- One major change that occurs as the body ages is a process
termed "thymic involution." ... As humans age, the thymus
naturally atrophies... Once the immune system fully develops and
can protect the host against a myriad of antigens, the thymus
may be too costly to maintain, so it is evolutionarily advantageous
to decrease the amount of thymic tissue and use the energy that
would have supported the thymus for other purposes. However, because
T cells play such a prominent role in immunity, longer-lived individuals
still need a continuous supply of "fresh" T cells to
protect against newly-encountered antigens, and this slow but
progressive loss of thymic tissue has profound effects on the
entire immune system of the aged.
Ref:
The
Immunology of Aging
Also
see: Thymic
involution tied to progression of pediatric HIV infection
Endocrine:
Uterus
(click on for all fluorinated
pesticides)
--
Fluazifop butyl was evaluated for developmental toxicity in adult
virgin Sprague-Dawley CD rats (160/group) administered oral doses
of 0, 1, 5, 10 and 200 mg/kg/day during gestation days 6-20. No
increased maternal mortality or overt toxicity was attributed
to treatment. At gestation Day 21 sacrifice of the dams, a slight
but significantly depressed mean bodyweight gain among those of
a 200 mg/kg/day dosage was indicative of dose-related and significant
(p < 0.05; Student's t-test) reduction in
gravid uterus weights... [ICI
AMERS INC; Teratology Study with Fluazifop Butyl in Rats; 06/03/81;
EPA Doc No. 88-920007020; Fiche No. OTS0545395]
-- Fluazifop butyl (CAS # 69806-50-4) was evaluated for developmental
toxicity in the progeny of Sprague-Dawley CD rats administered
oral doses of 0 (corn oil vehicle control), 10, 50 and 200 mg/kg/day
by gavage on gestation days 6-20. The general condition of treated
rats was comparable to controls. Treatment did not alter food
consumption and usage efficiency, although terminal mean bodyweights
were significantly (p < 0.05; multiple t-test) depressed in the
high-dose (200 mg/kg/day) group. Gravid
uterus weights were also significantly
(p < 0.01) elevated in association with the high dosage...
[ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate butyl: Effects of Oral Administration Upon Pregnancy
in Rats; 05/23/80; EPA Doc No. 88-920006839; Fiche No. OTS0543844]
Ref:
TOXNET profile from Hazardous Substances Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
Eye
(click on for all fluorinated pesticides)
-- Fluazifop-butyl
(CAS # 69806-50-4) was evaluated for subchronic oral toxicity
in beagle dogs (4/sex/group) administered doses of 0 (vehicle
control, corn oil), 5, 25 and 250 mg/kg/day in gelatin capsules
for 13 weeks. Severe corneal ulceration,
requiring humane sacrifice of 2 males and 1 female of a 250 mg/kg/day
dosage during Week 4, prompted adjustment of this regimen to 125
mg/kg/day for the remainder of study. Prior to sacrifice, these
dogs also exhibited progressive conjunctivitis
and photophobia, and all lost weight...
Histopathological evaluation confirmed treatment-related lesions
of the eyes, liver, testes and gastrointestinal
tract. [ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate: 13-Week Oral Toxicity Study in Beagle Dogs; 04/24/80;
EPA Doc No. 88-920006846; Fiche No. OTS0543851]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows... Eyes of eight high dose dogs
had cataracts, usually accompanied by miliary ("seed-like"
appearance) vacuolation of the lens... Possible adverse
effects (many-faceted toxicity, including mortalities, at 125
mg/kg/day). Acceptable. Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Fetotoxic
(click on for all fluorinated
pesticides)
-- Fetotoxicity
(delayed ossification and eye opacities) was also demonstrated
in New Zealand White rabbits (the LOEL was 30 mg/kg/day; the NOEL
was 10 mg/kg/day). EPA believes that there is sufficient evidence
for listing fluazifop butyl on EPCRA
section 313 pursuant to EPCRA section 313(d)(2)(B) based on the
available hepatic and developmental toxicity data for this chemical.
-- In a teratogenicity study in Sprague-Dawley rats exposed via
oral gavage, delayed ossification and an increased incidence of
hydroureter were observed in fetuses
(the fetotoxic LOEL was 5 mg/kg/day;
the NOEL 1 mg/kg/day) and a teratogenic LOEL of 200 mg/kg/day
(the NOEL was 10 mg/kg/day) was determined based on the incidence
of diaphragmatic hernia.
Ref: USEPA/OPP. Support Document for the
Addition of Chemicals from Federal Insecticide, Fungicide, Rodenticide
Act (FIFRA) Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993). As cited by US EPA in:
Federal Register: January
12, 1994. Part IV. 40 CFR Part 372. Addition of Certain Chemicals;
Toxic Chemical Release Reporting; Community Right-to-Know; Proposed
Rule.
--
Reproductive Effects: In a 3-generation reproductive study in
rats, effects included reductions in weight gain, fetal weight,
ossification, testicular weight, spleen weight, increased prostate
weight and gestational length. No Effect Level (NEL) was 1 mg/kg/day.
Fetotoxic effects seen in the rabbit,
including reduced fetal weight and reduced ossification at higher
doses. No Effect Level (NEL)
was 30 mg/kg/day in rabbits. The NEL for teratogenic effects is
at least 10/mg/day in the rat, with diaphragmatic hernia at higher
doses. Not teratogenic at highest dose tested in rabbits (90 mg/kg/day).
While fluazifop-p-butyl is fetotoxic
when fed to
pregnant rats, human exposure data has concluded that female formulation
workers are not at increased risk of fetotoxic effects when skin
protection measures are applied.
Ref: Material Safety Data Sheet for Fusilade
DX (active indredient Fluazifop-P-Butyl). Syngenta. January 21,
2002.
http://www.fluoridealert.org/pesticides/Fluazifop-P-Butyl.MSDS.pdf
Gastrointestinal
Tract (click
on for all fluorinated pesticides)
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study.
Fluazifop-butyl caused ulcerations in the
g.i. tract, specifically in the tongue, lip, mouth lining, stomach
pylorus, or cecum. Ulcerations may have contributed to
low RBC parameters in both sexes (reduced HCT, Hb levels, and
RBC counts, particularly in males)...
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
Hydroureter
4.2.3.2 Fluazifop-butyl - Sprague Dawley Rats In a developmental
toxicity study (MRID# 00088857, 92067047 and 92067019), fluazifop-butyl,
[PP009 (94.8% a.i., batch/lot # P14)] was administered to 22 female
CD Sprague Dawley strain rats/group in a corn oil (2 ml/kg) gavage
at dose levels of 0, 10, 50 or 200 mg/kg bw/day from days 6 through
20 of gestation. Animals were sacrificed on day 21 ... The
incidence of bilateral hydronephrosis at 200 mg/kg/day exceeded
the historical control mean but not the range. The
incidence of bilateral hydroureter at 200 mg/kg/day exceeded the
historical control range and mean. No hydronephrosis nor
hydroureter were seen in concurrent controls. In addition, subcutaneous
edema (17.7% at 200 mg/kg/day versus 3.4% in concurrent controls)
exceeded the mean of 8.9% in historical controls (the upper range
was unreadable). The concurrent controls also were less
than the mean of historical controls. Diaphragmatic hernia was
seen in 1 fetus at 10 mg/kg/day, none at 50 mg/kg/day, and in
3 fetuses at 200 mg/kg/day, with no incidences in 2970 historical
control fetuses. However a subsequent much
larger study (MRID# 00088858) with 160 litter/group, showed an
incidence of diaphragmatic hernias of 3/1113 in control fetuses,
1/1081 fetuses at 1 mg/kg/day, 3/1073 fetuses at 5 mg/kg/day,
2/1064 fetuses at 10 mg/kg/day, and a statistically significantly
increased incidence in 59/1064 fetuses and 45/159 litters at 200
mg/kg/day. Since this anomaly was seen at a higher incidence (3
fetuses) in the controls in this study and was not replicated
at the same dose in the second study, the single incidence of
diaphragmatic hernia at 10 mg/kg/day was considered to be an aberration
and not attributable to treatment. For developmental toxicity,
the LOAEL is 10 mg/kg/day based on incomplete and/or irregular
ossification of the cranial bones and incomplete ossification
of thoracic vertebral centra; a NOAEL was not established.
Ref:
December 10, 2004. US
EPA> Fluazifop-P-butyl: Revised HED Chapter of the Tolerance
Reassessment Eligibility Decision (TRED) Document. EPA Docket
number: OPP-2004-0347-0003
http://www.fluorideaction.org/pesticides/f-p-b.opp-2004-0347-0003.pdf
Kidney
(click on for all
fluorinated pesticides)
-- Fluazifop-butyl
(CAS # 69806-50-4) was evaluated for subchronic dietary toxicity
in Wistar rats (20/sex/group) receiving 0, 10, 100 and 2000 ppm
by dietary inclusion for 13 weeks. Mean achieved dosages during
13-week treatment were 0, 0.7, 7.1 and 144.5 mg/kg/day in males
of the respective treatment groups and 0, 0.8, 8.0 and 161.9 mg/kg/day
in females... Upon terminal necropsy, only high-dose males had
evidence of hepatic enlargement or swelling (7/20), although higher
absolute and relative liver weights were significant (p < 0.01,
Student's t-test) in both males and females of the 2000 ppm group.
Also, relative kidney weights were also
significantly elevated in the high-dose males. Histopathology
confirmed a specific liver toxicity in male rats, marked by significant
dose-related hepatocytic hypertrophy with isolated instances of
vesicular nuclei and or periacinal hepatocytic necrosis. Renal
tubular degeneration, 70% and 20% in 2000 and 100 ppm males respectively,
correlated with elevated kidney weights in these groups.
[ICI AMERICAS INC; 13 Week Dietary Toxicity Study with PP009 in
Rats (Final Report); 05/29/80; EPA Doc No. 88-920006943; Fiche
No. OTS0545346]
-- Fluazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2). Thirty days after sacrifice of their
offspring, the surviving F1 females and select F1 males were sacrificed
and with representative F1 and F2 offspring were examined histologically...
F1 parents were found with gross indications of toxicity.
Liver
and kidney
weights were significantly high (250
ppm) relative to controls, while spleen weights were low in both
sexes (80, 250 ppm).Upon necropsy, these F1 progeny were found
with dose-related increased hydronephrosis
[kidney]... Upon
histological investigation, increased incidence geriatric nephropathy
[kidney] (both sexes, 80 and 250 ppm), distension of mesenteric
and/or cervical lymph nodes (250 ppm) and increased severity of
nephrocalcinosis (females, 80 and
250 ppm), .. [ICI AMERS INC; Fluazifop butyl: Effects Upon Reproductive
Performance of Rats Treated Continuously Through 2 Generations
(Final Report); 03/17/81; EPA Doc No. 88-920006849; Fiche No.
OTS05543854]
--- Definition for nephrocalcinosis:
A form of renal stone disease where the kidney tissue is characterised
by foci of calcification in addition to numerous deposits of calcium
phosphate and calcium oxalate.
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
4.2.3.2 Fluazifop-butyl - Sprague Dawley Rats In a developmental
toxicity study (MRID# 00088857, 92067047 and 92067019), fluazifop-butyl,
[PP009 (94.8% a.i., batch/lot # P14)] was administered to 22 female
CD Sprague Dawley strain rats/group in a corn oil (2 ml/kg) gavage
at dose levels of 0, 10, 50 or 200 mg/kg bw/day from days 6 through
20 of gestation. Animals were sacrificed on day 21 ... The
incidence of bilateral hydronephrosis at 200 mg/kg/day exceeded
the historical control mean but not the range. The
incidence of bilateral hydroureter at 200 mg/kg/day exceeded the
historical control range and mean. No hydronephrosis nor
hydroureter were seen in concurrent controls. In addition, subcutaneous
edema (17.7% at 200 mg/kg/day versus 3.4% in concurrent controls)
exceeded the mean of 8.9% in historical controls (the upper range
was unreadable). The concurrent controls also were less
than the mean of historical controls. Diaphragmatic hernia was
seen in 1 fetus at 10 mg/kg/day, none at 50 mg/kg/day, and in
3 fetuses at 200 mg/kg/day, with no incidences in 2970 historical
control fetuses. However a subsequent much
larger study (MRID# 00088858) with 160 litter/group, showed an
incidence of diaphragmatic hernias of 3/1113 in control fetuses,
1/1081 fetuses at 1 mg/kg/day, 3/1073 fetuses at 5 mg/kg/day,
2/1064 fetuses at 10 mg/kg/day, and a statistically significantly
increased incidence in 59/1064 fetuses and 45/159 litters at 200
mg/kg/day. Since this anomaly was seen at a higher incidence
(3 fetuses) in the controls in this study and was not replicated
at the same dose in the second study, the single incidence of
diaphragmatic hernia at 10 mg/kg/day was considered to be an aberration
and not attributable to treatment. For developmental
toxicity, the LOAEL is 10 mg/kg/day based on incomplete and/or
irregular ossification of the cranial bones and incomplete ossification
of thoracic vertebral centra; a NOAEL was not established.
Ref:
December 10, 2004. US
EPA> Fluazifop-P-butyl: Revised HED Chapter of the Tolerance
Reassessment Eligibility Decision (TRED) Document. EPA Docket
number: OPP-2004-0347-0003
http://www.fluorideaction.org/pesticides/f-p-b.opp-2004-0347-0003.pdf
Liver
(click on for all fluorinated pesticides)
A 3-month rat feeding
study demonstrated hepatocyte hypertrophy in males (the LOEL was
5 mg/kg/day; the NOEL was 0.5 mg/kg/ day). In a 1-year feeding
study, dogs had changes in serum alkaline phosphatase and alanine
aminotransferase and/or alanine sulfatransferase (the LOEL was
25 mg/kg/day; the NOEL was 5 mg/kg/day). Similar changes were
also reported in dogs following 3 months exposure in their diet
(the LOEL was 125 mg/kg/day). In a carcinogenicity study, male
mice fed 20 ppm (2.6 mg/kg/day, the LOEL) had an increased incidence
of hepatocyte hypertrophy. The NOEL
was 5 ppm or 0.65 mg/kg/ day. Male and female mice exposed to
a higher dose of 80 ppm (10.4 mg/ kg/day) had increased
liver weight (relative and absolute) and hypertrophy of
periacinal hepatocytes. Males in this dose group also had increased
pigmentation in hepatocytes and Kupffer cells. In a teratogenicity
study in Sprague-Dawley rats exposed via oral gavage, delayed
ossification and an increased incidence of hydroureter were observed
in fetuses (the fetotoxic LOEL was 5 mg/kg/day; the NOEL 1 mg/kg/day)
and a teratogenic LOEL of 200 mg/kg/day (the NOEL was 10 mg/kg/day)
was determined based on the incidence of diaphragmatic hernia.
Maternal toxicity was observed in this study at doses higher than
those causing fetotoxicity and included reduced body weight gain
and decreased gravid uterus (the maternal LOEL was 200 mg/kg/day;
the NOEL was 10 mg/kg/day). In a 2-generation reproductive toxicity
dietary study in Wistar rats, the reproductive LOEL of 250 ppm
(12.5 mg/kg/day; the NOEL was 80 ppm or 4 mg/kg/day) was based
on reduced litter sizes, reduced viability, reduced testis and
epididymis weights and tubular atrophy in offspring. Fetotoxicity
(delayed ossification and eye opacities) was also demonstrated
in New Zealand White rabbits (the LOEL was 30 mg/kg/day; the NOEL
was 10 mg/kg/day). EPA believes that there
is sufficient evidence for listing fluazifop butyl on EPCRA section
313 pursuant to EPCRA section 313(d)(2)(B) based on the available
hepatic and developmental toxicity data for this chemical.
Ref: USEPA/OPP. Support Document for the
Addition of Chemicals from Federal Insecticide, Fungicide, Rodenticide
Act (FIFRA) Active Ingredients to EPCRA Section 313. U. S. Environmental
Protection Agency, Washington, DC (1993). As cited by US EPA in:
Federal Register: January 12, 1994. Part IV. 40 CFR Part 372.
Addition of Certain Chemicals; Toxic Chemical Release Reporting;
Community Right-to-Know; Proposed Rule.
http://www.epa.gov/tri/frnotices/59fr1788.htm
-- Fluazifop-butyl
(CAS # 69806-50-4) was evaluated for subchronic oral toxicity
in beagle dogs (4/sex/group) administered doses of 0 (vehicle
control, corn oil), 5, 25 and 250 mg/kg/day in gelatin capsules
for 13 weeks. Severe corneal ulceration, requiring humane sacrifice
of 2 males and 1 female of a 250 mg/kg/day dosage during Week
4, prompted adjustment of this regimen to 125 mg/kg/day for the
remainder of study... Distended gall bladders, large
friable livers and congested caecum and colon from engorgement
of the blood vessels were noted upon necropsy. Histopathological
evaluation confirmed treatment-related lesions of the eyes, liver,
testes and gastrointestinal tract. [ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate: 13-Week Oral Toxicity Study in Beagle Dogs; 04/24/80;
EPA Doc No. 88-920006846; Fiche No. OTS0543851]
-- Fluazifop-butyl (CAS # 69806-50-4) was evaluated for subchronic
dietary toxicity in Wistar rats (20/sex/group) receiving 0, 10,
100 and 2000 ppm by dietary inclusion for 13 weeks. Mean achieved
dosages during 13-week treatment were 0, 0.7, 7.1 and 144.5 mg/kg/day
in males of the respective treatment groups and 0, 0.8, 8.0 and
161.9 mg/kg/day in females... Upon terminal necropsy, only high-dose
males had evidence of hepatic enlargement or swelling (7/20),
although higher absolute and relative liver weights were significant
(p < 0.01, Student's t-test) in both males and females of the
2000 ppm group... Histopathology confirmed a specific liver
toxicity in male rats, marked by significant dose-related hepatocytic
hypertrophy with isolated instances of vesicular nuclei and or
periacinal hepatocytic necrosis... [ICI AMERICAS INC; 13 Week
Dietary Toxicity Study with PP009 in Rats (Final Report); 05/29/80;
EPA Doc No. 88-920006943; Fiche No. OTS0545346]
-- -- Fluazifop-butyl (CAS # 69806-50-4) was evaluated for subacute
oral toxicity in Wistar albino rats (10/sex/group) administered
10 ml/kg doses in corn oil of 0, 4, 20, 100 and 500 mg/kg/day
by oral gavage, 5 days/week for 2 weeks. Five-day weekly treatment
periods were each followed by 2-day recovery... Male rats of 100
and 500 mg/kg/day dosages had higher absolute and relative liver
weights that was not seen in their female counterparts. Microscopic
examination confirmed a particular liver
pathology of treated males, characterized by dosage-related hepatocytic
hypertrophy and necrosis (study lethalities), and dosage-related
slight to moderate periacinal hepatocytic hypertrophy... [ICI
AMERS INC; 14 Day Subacute Oral Toxicity Study with PP009 in Rats;
07/11/80; EPA Doc No. 88-920007017; Fiche No. OTS0545392]
-- Fluazifop butyl (CAS # 69806-50-4) was evaluated for developmental
toxicity in the progeny of Sprague-Dawley CD rats administered
oral doses of 0 (corn oil vehicle control), 10, 50 and 200 mg/kg/day
by gavage on gestation days 6-20... Relative
liver weights were also significantly (p < 0.05) greater
in high-dose dams... [ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate butyl: Effects of Oral Administration Upon Pregnancy
in Rats; 05/23/80; EPA Doc No. 88-920006839; Fiche No. OTS0543844]
-- Fluazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2). Thirty days after sacrifice of their
offspring, the surviving F1 females and select F1 males were sacrificed
and with representative F1 and F2 offspring were examined histologically...
Liver and kidney weights were
significantly high (250 ppm) relative to controls, while spleen
weights were low in both sexes (80, 250 ppm)... [ICI AMERS INC;
Fluazifop butyl: Effects Upon Reproductive Performance of Rats
Treated Continuously Through 2 Generations (Final Report); 03/17/81;
EPA Doc No. 88-920006849; Fiche No. OTS05543854]
-- Fluazifop-butyl
(CAS # 69806-50-4) was evaluated for subchronic oral toxicity
in beagle dogs (4/sex/group) administered doses of 0 (vehicle
control, corn oil), 5, 25 and 250 mg/kg/day in gelatin capsules
for 13 weeks. Severe corneal ulceration,
requiring humane sacrifice of 2 males and 1 female of a 250 mg/kg/day
dosage during Week 4, prompted adjustment of this regimen to 125
mg/kg/day for the remainder of study. Prior to sacrifice... Histopathological
evaluation confirmed treatment-related lesions of the
eyes, liver,
testes and gastrointestinal tract. [ICI AMERS INC; Butyl 2-(4-(5-trifluoromethyl-2-pyridyloxy)phenoxy)
propionate: 13-Week Oral Toxicity Study in Beagle Dogs; 04/24/80;
EPA Doc No. 88-920006846; Fiche No. OTS0543851]
Ref: Fluazifop-butyl. TOXNET profile from
Hazardous Substances Data Bank.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
**411-083 069476 Virgo,
D. M., "Fluazifop-butyl: 55 week oral toxicity study in beagle
dogs," Life Science Research, Stock, Essex, England, 10/15/82.
LSR Report No. 81/ILK019/620. Six dogs/sex/group were dosed for
55 weeks with Fluazifop-butyl, 99.6% purity, by gelatin capsules
at dose levels of 0, 5, 25, and 125 mg/kg/day in a chronic study.
Test article within capsules was dissolved in 0.4 ml/kg corn oil
vehicle. NOEL = 5 mg/kg/day... All
other noteworthy findings were limited to the high dose group,
as follows. Seven 125 mg/kg/day dogs (5 M, 2 F) were killed in
extremis prior to term, generally after at least 29 weeks on study...
Liver dysfunction was indicated by periacinar hepatocytic degeneration
and thinning of hepatic cords in some dogs, other hepatocytic
changes such as vacuolation and/or granular cytoplasm, and occasional
bile plugs in the canaliculi •.
Most clinical chemistry changes were plausibly related to liver
toxicity, including elevated alkaline phosphatase, ALT, and occasionally
AST. Substantially increased BSP retention was consistent
with biliary disturbance. Urine was typically bright yellow or
orange due to high bile pigment concentrations. Cholesterol
was consistently reduced. Male reproductive toxicity included
testicular tubular degeneration and reduced/absent spermatozoa
in epididymides. Possible adverse effects (many-faceted
toxicity, including mortalities, at 125 mg/kg/day). Acceptable.
Aldous, 5/20/02.
Ref: May 20, 2002. SUMMARY OF TOXICOLOGY
DATA FLUAZIFOP-P-BUTYL. California EPA. Department of Pesticide
Regulation. Medical Toxicology Branch.
http://www.fluoridealert.org/pesticides/Fluazifop-P-butyl.CAepa.02.pdf
• Canaliculi
- In liver, small channels between hepatocytes
through which bile flows to the bile duct and thence to the intestinal
lumen.
Abstract:
The
aim of this study was to determine the effect of herbicide fluazifop,
on the early occurring changes in rat liver regarded as hepatic
markers of peroxisome proliferators (PPs). Fluazifop was administered
orally to male Wistar rats at increasing doses from 5.6 to 891
mg/kg body weight per day for 1, 2, 4, 7 and 14 consecutive days
and peroxisome proliferation, induction of some peroxisome-associated
enzymes and mitogenesis (S-phase, M-phase and percentage of binucleated
hepatocytes) were studied. Short-term treatment
of rats with fluazifop resulted in hepatomegaly due to time dependent
proliferation of smooth endoplasmic reticulum (SER) and peroxisomes.
The increase in the number of peroxisomes in the hepatocytes
was supported by an increase in peroxisomal palmitoyl-CoA oxidation
and catalase activity. In contrast to other PPs fluazifop induced
low rate of rcplicative DNA synthesis and did not affect mitoses
(M-phase). DNA synthesis was accompanied by the appearance of
binucleated hepatocytes. Thus, we can conclude
that fluazifop produces in male Wistar rats hepatomegaly due to
cellular hypertrophy. The threshold dose for palmitoyl-CoA
oxidation and DNA synthesis was 112 and 223 mg/kg body weight
per day, respectively. The value for hepatomegaly and catalase
activity was 56 mg/kg body weight per day. The
results presented in this paper demonstrated that fluazifop can
be classified as a weak rodent PPs.
Ref:
Hepatocellular peroxisome proliferation and DNA synthesis in Wistar
rats treated with herbicide fluazifop; by Kostka G, Palut D, Ludwicki
JK, Kopec-Szlezak J, Wiadrowska B, Lembowicz K. Toxicology. 2002
Sep 16;178(3):221-8.
Mesenteric
Lymph Nodes (click
on for all fluorinated pesticides)
Fluazifop-butyl (CAS # 69806-50-4) was evaluated for subacute
oral toxicity in Wistar albino rats (10/sex/group) administered
10 ml/kg doses in corn oil of 0, 4, 20, 100 and 500 mg/kg/day
by oral gavage, 5 days/week for 2 weeks. Five-day weekly treatment
periods were each followed by 2-day recovery. High-dose males
only displayed overt toxicity and comprised the 2 study lethalities.
These two 500 mg/kg/day males were sacrificed in extremis on Days
11 and 14 with severe pharmacotoxic signs including piloerection,
reduced motor activity, retinal pallor, and a prone or hunched
posture. One rat was bleeding from the penis ... The decedent
animals were found with abnormal upper gastrointestinal contents,
and one rat exhibited congestion of mesenteric
lymph nodes with pallor of kidneys, liver and spleen...
[ICI AMERS INC; 14 Day Subacute Oral Toxicity Study with PP009
in Rats; 07/11/80; EPA Doc No. 88-920007017; Fiche No. OTS0545392].
-- Fuazifop butyl was evaluated for reproductive and developmental
effects in 2 successive generations of Charles River Wistar strain
rats (30/sex/group) exposed continuously to 0, 10, 80 and 250
ppm in the diet. Each respective parent generation had received
treatment for a minimum of 100 days (F0) and 120 days (F1) prior
to mating. F0 and F1 dams weaned their progeny for 25 days postpartum
to time of offspring selection for mating and continued study
(F1) or sacrifice (F1, F2)... Upon histological investigation,
increased incidence geriatric nephropathy (both sexes, 80 and
250 ppm), distension of mesenteric and/or
cervical lymph nodes (250 ppm) and increased severity of
nephrocalcinosis (females, 80 and 250 ppm), and an increased slight
testicular tubular atrophy in males (250 ppm) were noted. F2 female
progeny of 250 ppm exposed parents were found with significantly
increased absolute and relative spleen weights. Histological changes
were limited to marginal increases in hydronephrosis. [ICI AMERS
INC; Fluazifop butyl: Effects Upon Reproductive Performance of
Rats Treated Continuously Through 2 Generations (Final Report);
03/17/81; EPA Doc No. 88-920006849; Fiche No. OTS05543854]
Ref: TOXNET profile from Hazardous Substances Data Bank. FLUAZIFOP-BUTYL.
CASRN: 69806-50-4.
http://www.fluoridealert.org/pesticides/fluazifop-butyl.toxnet.hsdb.htm
Spleen
(click on for all fluorinated
pesticides)
Fluazifop butyl was
evaluated for reproductive and developmental effects in 2 successive
generations of Charles River Wistar strain rats (30/sex/group)
exposed continuously to 0, 10, 80 and 250 ppm in the diet. Each
respective parent generation had received treatment for a minimum
of 100 days (F0) and 120 days (F1) prior to mating. F0 and F1
dams weaned their progeny for 25 days postpartum to time of offspring
selection for mating and continued study (F1) or sacrifice (F1,
F2). Thirty days after sacrifice of their offspring, the surviving
F1 females and select F1 males were sacrificed and with representative
F1 and F2 offspring were examined histologically.... F2 female
progeny of 250 ppm exposed parents were found with significantly
increased absolute and relative spleen weights... [ICI
AMERS INC; Fluazifop butyl: Effects Upon Reproductive Performance
of Rats Treated Continuously Through 2 Generations (Final Report);
03/17/81; EPA Doc No. 88-920006849; Fiche No. OTS05543854]
Ref: TOXNET profile from Hazardous Substances
Data Bank for FLUAZIFOP-BUTYL.
http://www.fluoridealert.org/pesticides/Fluazifop-butyl.TOXNET.HSDB.htm
Teratogenic
(click on for all
fluorinated pesticides)
In a teratogenicity
study in Sprague-Dawley rats exposed via oral gavage, delayed
ossification and an increased incidence of hydroureter
were observed in fetuses (the fetotoxic LOEL was 5 mg/kg/day;
the NOEL 1 mg/kg/day) and a teratogenic
LOEL of 200 mg/kg/day (the NOEL was 10 mg/kg/day) was determined
based on the incidence of diaphragmatic
hernia.
Ref:
USEPA/OPP. Support Document for the Addition of Chemicals from
Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) Active
Ingredients to EPCRA Section 313. U. S. Environmental Protection
Agency, Washington, DC (1993).
As cited by US EPA in: Federal
Register: January 12, 1994. Part IV. 40 CFR Part 372. Addition
of Certain Chemicals; Toxic Chemical Release Reporting; Community
Right-to-Know; Proposed Rule.
|
Environmental
(click on
for all fluorinated pesticides)
Highly
toxic to Zooplankton.
Ref: Pesticide
Action Network Acute Aquatic Ecotoxicity Summaries.
--
Environmental Bioconcentration:
An estimated BCF of 1500 was calculated for fluazifop-butyl(SRC),
using a measured log Kow of 4.5(1) and a recommended regression-derived
equation(2). According to a classification scheme(3),
this BCF suggests that bioconcentration in aquatic organisms
is very high(SRC).
--
Environmental Fate/Exposure Summary:
...
If released into water, this compound is expected to adsorb
strongly to suspended solids and sediment in the water column
based on its estimated Koc. Volatilization from water surfaces
is not expected to be an important fate process based on
the estimated Henry's Law constant for this compound. The
potential for bioconcentration in aquatic organisms is very
high based on an estimated BCF of 1500. Hydrolysis half-lives
of 2.2 years and 79 days have been estimated for fluazifop-butyl
at pHs 7 and 8, respectively. Abiotic hydrolysis
of fluazifop-butyl has been observed to be catalyzed by
soil colloids. (SRC)
-- AQUATIC FATE: ... According
to a classification scheme(5), an estimated BCF of 1500(3,SRC),
from a measured log Kow(2), suggests
that bioconcentration in aquatic organisms is very high(SRC).
Biodegradation of fluazifop-butyl
in aquatic systems may be important(SRC), given its microbial
degradation in moist soils(6). Hydrolysis half-lives of
2.2 years and 79 days have been estimated for fluazifop-butyl
at pHs 7 and 8, respectively(7)...
--
TERRESTRIAL FATE:
... Biodegradation of fluazifop-butyl in soil is expected
to be important(SRC); fluazifop-butyl is rapidly biodegraded
in moist soils, with a half-life of less than 1 week; the
major degradation product being fluazifop(4).
--
Soil Adsorption/Mobility:
... Fluazifop-butyl is of low mobility in soil(4). Fluazifop-butyl
has been found to bind strongly with homoionic clays(5).
Ref:
Hazardous Substance Data Bank for FLUAZIFOP-BUTYL CASRN:
69806-50-4 from Toxnet.
|
|

