|
Return to Dichlorodifluoromethane
Index Page
ACTIVITY: Insecticide,
Fungicide Propellant, EPA List 2 Inert (halogenated organic)
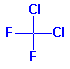
Structure:
Adverse Effects:
Ataxia
Brain
CNS
Heart
Leukemia
Liver
Lung
Tremors/Convulsions
Environmental
| As
of September 27, 2003, Dichlorodifluoromethane is listed by
US EPA as a List 2 Inert in pesticidal formulations. See:
http://www.epa.gov/opprd001/inerts/inerts_list2.pdf
Accidental
death of child playing with deodorant aerosol
Source: Lancet; VOL 1 ISS Apr 8 1978
Author: Jefferson IG
Author Address: Westminister Children's Hosp., London SW1, England
Comments: Letters Abstract: IPA COPYRIGHT: ASHP A 4-yr-old boy,
playing with an antiperspirant deodorant in the bathtub, inhaled
the propellants, 50.5% trichloromonofluoromethane (fluorocarbon
11) and 43% dichlorodifluoromethane (fluorocarbon
12), became deeply unconscious with no spontaneous respiration,
and no cerebral activity, and died 5 days later.
Body
Burden: In a pilot study of pollutants in the milk
of women living in 4 urban-industrial areas in the
US, dichlorodifluoromethane was identified, not quantified,
in 2 of 8 samples(1).
[(1) Pellizzari ED et al; Bull Environ Contam
Toxicol 28: 322-8 (1982)]
Manufacturing/Use
Information: Major Uses:
The active
ingredient is no longer contained in any registered pesticide
products ... "cancelled." [United States Environmental Protection
Agency/ Prevention, Pesticides and Toxic Substances; Status
of Pesticides in Registration, Reregistration, and Special
Review. (1998) EPA 738-R-98-002 312] -
Note from FAN: An "Inert"
used in a pesticidal formulation is not considered an "active
ingredient."
Fully
halogenated chlorofluorocarbons (CFCs) such as dichlorodifluoromethane
were scheduled for production phase-out in 1987 by the Montreal
Protocol. Although originally scheduled for 50% production
phase-out by the year 2000 in developed countries, the worsening
ozone depletion has forced acceleration of the CFC phase-out.
Leak-detecting
agent; freezing of foods by direct contact; /SRP: former use/
chilling of cocktail glasses, refrigerant in air conditioners,
plastics, blowing agent, solvent
To prepare
frozen tissue sections /former use/
In various
"skin freezes" by aerosol application, as propellant for antibiotic
powders, mastitis formulations, etc, and for admixture to
other gases such as ethylene oxide to make them non-flammable.
/former use/
Manufacturers:
Allied Signal Inc. - Prod site:
Danville, IL 61834
Dupont Chemical Inc. - Prod sites:
Antioch, CA 94509; Montague, MI 49437.
Elf Atochem North America Inc. - Prod
site: Calvert City, KT 42029.
| Dichlorodifluoromethane
concn in room air as a result of release of aerosol can
products. Level at periods after 60 sec release of hair
spray in a 29.3 cu m room (in mg/cu m): during
release: 306.8; 30 min after: 12.4; 60 min after: 0.5.
Level at periods after 30 second release of
insect spray in a 21.4 cu m room (in mg/cu m):
1 min: 2,304.0; 60 min: 130.4; 150 min: 56.8.
/From table/ [USEPA; Ambient Water Quality Criteria Doc:
Halomethanes p.C-19 (1980) EPA 440/5-80-051] |
Ref:
Dichlorodifluoromethane. TOXNET profile from Hazardous Substances
Data Bank. http://www.fluorideaction.org/pesticides/dichlorodifluorometh.toxnet.htm |
Freon
12. Standards for Inhalation Exposure
A. Occupational Exposure Limits (NIOSH, 1997; ACGIH,
1994).
|
1.
Ceiling Limit (C) (not to be exceeded at any time):
|
Not
established.
|
2.
Short-Term Exposure Limit (STEL or ST):
|
Not
established.
|
3.
8-Hour Time Weighted Average (TWA):
|
1,000 ppm (4,950 mg/m 3 )
|
4.
10-Hour Time Weighted Average (TWA):
|
1,000 ppm (4,950 mg/m 3 )
|
5.
Immediately Dangerous to Life & Health (IDLH):
|
15,000 ppm (74,250 mg/m 3 )
|
Freon
12. Acute
Reference Exposure Levels (1-hour exposure)
(OEHHA, 1999)
|
1.
Level protective against mild adverse effects:
|
Not
established
|
2.
Level protective against severe adverse effects:
|
Not
established
|
3.
Level protective against mild adverse effects:
|
Not
established
|
Freon
12. Chronic
Reference Exposure Level (multiple years)
(OEHHA, 2002A)
|
Level
protective of adverse health effects:
|
Not
established.
|
Freon
12. Chronic
Reference Concentration (lifetime exposure)
(IRIS, 2003)
|
Level
protective of adverse health effects:
|
Not
established.
|
Freon
12.
Preliminary Remediation Goals
(U.S. EPA, 2002, Region IX): |
for
Residential Soil
|
94
mg/kg
|
for Tap
Water
|
390
ppb (0.4 mg/l)
|
for Ambient
Air
|
0.21
mg/m 3
|
Ref:
September
24, 2003 (Revised). Released November 7, 2003)
- FREON [11, 12,
113]. Technical Support Document: Toxicology. Clandestine
Drug Labs/ Methamphetamine. Volume 1, Number 11. California
EPA, Office of Environmental Health Hazard Assessment (OEHHA),
Department of Toxic Substances Control.
|
Ataxia
(click on for all fluorinated
pesticides)
DOGS, MONKEYS, & GUINEA
PIGS EXPOSED TO 20% OF GAS IN AIR FOR SEVERAL HR A DAY FOR SEVERAL
DAYS SHOWED TEMPORARY INTOXICATION WITH TREMORS, ATAXIA,
AND TENDENCY TO STARE, SALIVATE, & LACRIMATE, BUT NO CUMULATIVE
TOXIC EFFECT & NO SPECIFIC OCULAR DISTURBANCE. [Grant, W.M. Toxicology
of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher,
1986. 322]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Brain
(click
on for all fluorinated pesticides)
Chronic
effects ... In the occupational setting, chronic fluorocarbon
exposure has been associated with a syndrome of impaired psychomotor
speed, impaired memory and learning,
and emotional instability (Reprotext, 2003). Repeated or prolonged
skin contact may cause dermatitis (NIOSH, 2001E; NIOSH, 2001D).
Ref:
September 24, 2003 (Revised)
- FREON [11, 12, 113].
Technical Support Document: Toxicology. Clandestine
Drug Labs/ Methamphetamine. Volume 1, Number 11. California EPA,
Office of Environmental Health Hazard Assessment (OEHHA), Department
of Toxic Substances Control.
There is a
significant accumulation of fluorocarbons in brain, liver
and lung compared to blood levels, signifying a tissue distribution
of fluorocarbons similar to that of chloroform. /Fluorocarbons/
[Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene
and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th
ed. New York, NY: John Wiley & Sons Inc., 1993-1994. 1203]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
CNS
(click
on for all fluorinated pesticides)
-- Health
Hazards - General. ...At high concentrations, Freon vapor
may cause pulmonary edema and neurological problems such as
central nervous system depression, dizziness, headache,
drowsiness, tremors, seizures, confusion, in-coordination, loss
of consciousness, and paralysis (Hazardtext, 2003B; Dupont, 1996A;
OSHA, 1998; NIOSH, 2003C).
-- Predisposing Conditions. Individuals
with pre-existing diseases of the central
nervous or cardiovascular system may have increased susceptibility
to the effects of Freons (Dupont, 1996A; OSHA, 1998; Dupont, 1996B;
Dupont, 1996D). Persons exposed to epinephrine or other sympathomimetic
amines, e.g., bronchodilators and nasal decongestants (e.g., Sudafed
•), might be at increased risk for the cardiotoxic effects of
Freons (Reprotext, 2003).
-- Special Concerns for Children.
Children may inhale relatively larger doses of Freon because,
relative to their body weight, they have a greater lung surface
area and larger minute volume than adults. Since Freon has a high
vapor density, children could also receive high doses due to their
short stature and the higher levels of Freon vapor that may be
present near the ground when Freon is spilled.
Ref:
September 24, 2003 (Revised)
- FREON [11, 12, 113].
Technical Support Document: Toxicology. Clandestine
Drug Labs/ Methamphetamine. Volume 1, Number 11. California EPA,
Office of Environmental Health Hazard Assessment (OEHHA), Department
of Toxic Substances Control.
Heart
(click
on for all fluorinated pesticides)
--
11 subjects (7 being maintenance technicians of large cooling
and refrigerating systems) were exposed for 130 min to CFC-12
(weighted exposure 0.46, 49.9, and 87.7 g/cu m. ... This led to
acute reduction of ventilatory lung capacity only at the two highest
CFC-12 concentrations, under which conditions a significant
decrease in the heart frequency was also observed. [WHO;
Environmental Health Criteria 113: Fully Halogenated Chlorofluorocarbons
p.90 (1990)]
-- Ten subjects /were exposed/ to CFC-11, CFC-12, CFC-114, two
mixtures of CFC-11 and CFC-12, and a mixture of CFC-12
and CFC-114 (breathing concentrations between 16 and 150
g/cu m) for 15, 45, or 60 seconds, and found significant acute
reduction of ventilatory lung capacity (FEV50, FEF25) on exposure
to each chlorofluorocarbon, as well as bradycardia and increased
variability in heart rate in seven subjects, negative T-waves
in two subjects (one was exposed to CFC-11 and CFC-12), and atrioventricular
block in 1 subject (CFC-114). Mixtures exerted stronger respiratory
effects than individual chlorofluorocarbon at the same
level of exposure. [WHO; Environmental Health Criteria 113: Fully
Halogenated Chlorofluorocarbons p.90 (1990)]
-- Deaths resulting
from cardiovascular collapse after
arrhythmias have been reported after inhalation of Freons
11 and 12. [Ellenhorn, M.J. and D.G.
Barceloux. Medical Toxicology - Diagnosis and Treatment of Human
Poisoning. New York, NY: Elsevier Science Publishing Co., Inc.
1988. 528]
Ref:
Profile from Hazardous Substances Data Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
-- Health
Hazards - General ... Inhalation of high concentrations
may also result in temporary alteration
of the heart’s electrical activity by increasing the sensitivity
of the heart to the arrhythmogenic action of epinephrine, causing
irregular pulse, palpitations, or inadequate circulation
(Dupont, 1996A; Dupont, 1996F; OSHA, 1998; Reprotext, 2003). Deliberate
inhalation (“sniffing”) may cause death without warning (Dupont,
1996A; Dupont, 1996F; OSHA, 1998).
-- Acute Effects ... Inhalation of
high concentrations (~5,000 ppm) is associated with the development
of arrhythmias and sudden death due to myocardial
sensitization to endogenous catecholamines (e.g., epinephrine).
-- Predisposing Conditions. Individuals
with pre-existing diseases of the central nervous or cardiovascular
system may have increased susceptibility to the effects
of Freons (Dupont, 1996A; OSHA, 1998; Dupont, 1996B; Dupont, 1996D).
Persons exposed to epinephrine or other sympathomimetic amines,
e.g., bronchodilators and nasal decongestants (e.g., Sudafed •),
might be at increased risk for the cardiotoxic
effects of Freons (Reprotext, 2003).
-- Special Concerns for Children.
Children may inhale relatively larger doses of Freon because,
relative to their body weight, they have a greater lung surface
area and larger minute volume than adults. Since Freon has a high
vapor density, children could also receive high doses due to their
short stature and the higher levels of Freon vapor that may be
present near the ground when Freon is spilled.
Ref:
September
24, 2003 (Revised)
- FREON [11, 12, 113].
Technical Support Document: Toxicology.
Clandestine Drug Labs/ Methamphetamine. Volume 1, Number 11. California
EPA, Office of Environmental Health Hazard Assessment (OEHHA),
Department of Toxic Substances Control.
Leukemia
(click on for all fluorinated pesticides)
-- Dichlorodifluoromethane
... /was/ tested by inhalation on Sprague-Dawley rats and Swiss
mice. The animals were exposed for 4 hr a day, 5 days a week;
rats were exposed for 104 weeks, and mice were exposed for 78
weeks. Animals were observed until spontaneous death. Exposure
of rats to dichlorodifluoromethane resulted in no noticeable differences
in the incidence of total benign and malignant tumors, and of
the most frequently expected or rate of tumors. Exposure of mice
to dichlorodifluoromethane resulted in a higher number of total
tumors in males and females which was dose related in males, pulmonary
adenomas in males and females at 5000 ppm, and leukemias
in males at 5000 and 1000 ppm and in
females at 1000 ppm. [Maltoni C et al; Annals of the New
York Academy of Sciences 534: 261-82 (1988)]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Liver
(click
on for all fluorinated pesticides)
-- Pathologic liver
changes were reported in guinea pigs chronically exposed (continued
for 90 days; or eight hr daily, 5 days weekly, for six wk) to
F 12 at levels of about 4,000 mg/cu
m (0.08 % by vol). [USEPA; Ambient Water Quality Criteria Doc:
Halomethanes p.C-52-5 (1980) EPA 440/5-80-051]
-- Short-term inhalation studies have been reported for CFC-11,
CFC-12, CFC-112, CFC-113, CFC-114,
and CFC-115. The results showed low toxicity, and the effects
observed were related mainly to the CNS,
respiratory tract,
and the liver. Oral toxicity
studies have confirmed the low toxicity. [WHO; Environmental Health
Criteria 113: Fully Halogenated Chlorofluorocarbons p.18 (1990)]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Lung
(click
on for all fluorinated pesticides)
-- 11 subjects (7 being
maintenance technicians of large cooling and refrigerating systems)
were exposed for 130 min to CFC-12 (weighted
exposure 0.46, 49.9, and 87.7 g/cu m. ... This led to
acute reduction of ventilatory lung capacity only at the
two highest CFC-12 concentrations, under which conditions a significant
decrease in the heart frequency was also observed. [WHO; Environmental
Health Criteria 113: Fully Halogenated Chlorofluorocarbons p.90
(1990)]
-- Ten subjects /were exposed/ to CFC-11,
CFC-12, CFC-114, two mixtures of CFC-11 and CFC-12, and
a mixture of CFC-12 and CFC-114 (breathing
concentrations between 16 and 150 g/cu m) for 15, 45, or 60 seconds,
and found significant acute reduction of
ventilatory lung capacity (FEV50, FEF25) on exposure to
each chlorofluorocarbon, as well as bradycardia
and increased variability in heart rate in seven subjects, negative
T-waves in two subjects (one was exposed to CFC-11 and CFC-12),
and atrioventricular block in 1 subject (CFC-114). Mixtures
exerted stronger respiratory effects than individual chlorofluorocarbon
at the same level of exposure. [WHO; Environmental Health
Criteria 113: Fully Halogenated Chlorofluorocarbons p.90 (1990)]
-- Dichlorodifluoromethane
... /was/ tested by inhalation on Sprague-Dawley rats and Swiss
mice. The animals were exposed for 4 hr a day, 5 days a week;
rats were exposed for 104 weeks, and mice were exposed for 78
weeks. Animals were observed until spontaneous death. Exposure
of rats to dichlorodifluoromethane resulted in no noticeable differences
in the incidence of total benign and malignant tumors, and of
the most frequently expected or rate of tumors. Exposure of mice
to dichlorodifluoromethane resulted in a higher number of total
tumors in males and females which was dose related in males, pulmonary
adenomas in males and females at 5000 ppm, and leukemias
in males at 5000 and 1000 ppm and in
females at 1000 ppm. [Maltoni C et al; Annals of the New
York Academy of Sciences 534: 261-82 (1988)]
-- Short-term inhalation studies have been reported for CFC-11,
CFC-12, CFC-112, CFC-113, CFC-114,
and CFC-115. The results showed low toxicity, and the effects
observed were related mainly to the CNS,
respiratory tract,
and the liver. Oral toxicity
studies have confirmed the low toxicity. [WHO; Environmental Health
Criteria 113: Fully Halogenated Chlorofluorocarbons p.18 (1990)]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Mutagenic
(click
on for all fluorinated pesticides)
Significant mutagenic
activity of F 12 at 2.47x10+6 mg/cu
m (50%) in air in a Neurospora crassa test system. [USEPA; Ambient
Water Quality Criteria Doc: Halomethanes p.C-59 (1980) EPA 440/5-80-051]
---- Note from FAN: Dichlorodifluoromethane
is also called Freon 12 or F 12.
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Tremors/Convulsions
(click
on for all fluorinated pesticides)
-- Inhalation of Freon
compounds at moderate concentrations initially produces CNS anesthetic
effects of intoxication and loss of psychomotor coordination.
In humans, this effect occurred at levels of 2500 ppm for Freon
113 and 10,000 ppm for Freon 12.
Higher concentrations produce marked in coordination, slurring
of speech, apprehension, and finally descreasing levels of consciousness.
Attendent hypoxia at high concentrations may also produce tremors,
convulsions, and cerebral edema.
Cardiac sensitization occurs at higher concentrations than initial
CNS intoxication. [Haddad, L.M., Clinical Management of Poisoning
and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co.,
1990. 1281]
-- .. Dogs, monkeys, rats, rabbits & guinea pigs /had continuous
exposure/ to 810 ppm of FC 12, 24
hr daily for 90 days. No deaths were attributed to exposure and
pathologic changes ... occurred only in guinea pigs, who showed
microscopic liver injury. ... At 200,000 ppm, guinea pigs, dogs
and monkeys exposed some 40 hr weekly for 10-12 weeks showed generalized
tremors and other signs of mild /CNS
depression/, as well as slight blood changes, but no pathologic
effects. [American Conference of Governmental Industrial Hygienists.
Documentation of the Threshold Limit Values and Biological Exposure
Indices. 5th ed. Cincinnati, OH:American Conference of Governmental
Industrial Hygienists, 1986.]
-- DOGS, MONKEYS, & GUINEA PIGS EXPOSED TO 20% OF GAS IN AIR FOR
SEVERAL HR A DAY FOR SEVERAL DAYS SHOWED TEMPORARY INTOXICATION
WITH TREMORS, ATAXIA, AND TENDENCY
TO STARE, SALIVATE, & LACRIMATE, BUT NO CUMULATIVE TOXIC EFFECT
& NO SPECIFIC OCULAR DISTURBANCE. [Grant, W.M. Toxicology of the
Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986.
322]
Ref: Profile from Hazardous Substances Data
Bank for Dichlorodifluoromethane.
http://www.fluoridealert.org/pesticides/Dichlorodifluorometh.TOXNET.htm
Environmental
(click
on for all fluorinated pesticides)
US
EPA: Class 1 Ozone-Depleting Substance. Lifetime of Global
Warming Potential: 100 years
Ref:
http://www.epa.gov/ozone/ods.htmlEnvironmental
Contamination Concerns
A.
Surface Water.
Volatilization from water surfaces is expected to be an
important fate process with estimated volatilization half-lives
for a model river and a model lake being four hours and
five days, respectively. Hydrolysis is not expected to occur.
Bioconcentration in organisms is low to moderate; BCF (Bioconcentration
factor: the ratio of the chemical concentration in the organism
to that in surrounding water) is from 11-86. Biodegradation,
adsorption to sediment, and abiotic degradation are insignificant.
Large volumes of Freon may sink to the bottom and gradually
bubble up to the surface if the water is not too cold (Hazardtext,
2003B; HSDB, 2001A; HSDB, 2001B).
B. Groundwater. In general,
Freons that are spilled onto soil have the
potential to leach into groundwater, because they
do not bind well to soil (Hazardtext, 2003B; HSDB, 2001A;
HSDB, 2001B). Fully halogenated hydrocarbons such as Freons
11, 12, and 113 are very resistant
to chemical and biological degradation and are likely to
be persistent contaminants if they reach groundwater.
D. Soil. If Freon is spilled
onto soil, a portion may evaporate from the surface and
the remainder will leach downward into the soil. Mobility
through the soil is expected to be moderate based on estimated
Koc values. Freon does not bind well to soil, and leaching
to groundwater is possible (Hazardtext, 2003B; HSDB,
2001B).
E. Air. Once released to air,
Freon exists solely as a gas. In the atmosphere, fully halogenated
Freons diffuse to the troposphere, where they are very stable
and can be transported great distances. Wet deposition may
result in some loss, but re-volatilization
into the atmosphere is likely. The only degradation
process is diffusion to the stratosphere, where photolytic
destruction of Freons results in depletion
of stratospheric ozone, thereby increasing the amount
of ultraviolet-B (UV-B) radiation reaching the earth’s surface
(Hazardtext, 2003B; HSDB, 2001A; HSDB, 2001B).
Ref:
September
24, 2003 (Revised). Released November 7, 2003)
- FREON [11, 12,
113]. Technical Support Document: Toxicology. Clandestine
Drug Labs/ Methamphetamine. Volume 1, Number 11. California
EPA, Office of Environmental Health Hazard Assessment (OEHHA),
Department of Toxic Substances Control.
Many
gases emitted as a result of industrial and agricultural
activities can accumulate in the earth's atmosphere and
ultimately contribute to alterations in the vertical distribution
and concentrations of stratospheric ozone. Among the most
important are those trace gases that have long residence
times in the atmosphere. This allows accumulation in the
troposphere and a gradual upward migration of the gases
into the stratosphere where they contribute to depletion
of stratospheric ozone layer. The atmospheric and chemical
processes involved are extremely complex. Trace
gases of particular concern include certain long
lived chlorofluorocarbons, such as CFC-11, CFC-12,
and CFC-113. Since the transport of these gases to the stratosphere
is slow, their residence times there are long, and the removal
processes are slow, any effect on stratospheric ozone already
seen is probably the result of anthropogenic emissions of
these gases several decades ago. Those gases already in
the atmosphere will continue to exert stratospheric ozone
depletion effects well into the next century. /Chlorofluorocarbons/
[WHO; Environmental Health Criteria 113: Fully Halogenated
Chlorofluorocarbons p.47 (1990)]
-- The realization that certain chlorofluorocarbons can
accumulate in the upper atmosphere and deplete the earth's
ozone layer has had a major impact on chemicals like dichlorodifluoromethane
which are used in large quantities and have the stability
to reach the stratosphere. Uses such as propellants in aerosols
which had accounted for about 75% of the release of dichlorodifluoromethane
and trichlorofluoromethane, the chemicals of greatest concern
(refrigerants and foams accounted for about 14 and 12%,
respectively), were banned in the US after Dec 15, 1978(1).
Previously dichlorodifluoromethane was the principal propellant
for non-food aerosols(1) and 60% of dichlorodifluoromethane
and trichlorofluoromethane production went into aerosols(1).
[(1) Smart BE; Kirk Othmer's Encycl Chem Tech 3rd NY,NY:
Wiley Interscience 10: 829-70 (1980)]
Ref:
Profile from Hazardous Substances Data Bank for Dichlorodifluoromethane.
http://www.fluorideaction.org/pesticides/dichlorodifluorometh.toxnet.htm
|
|

