|
Return to Cloransulam-methyl
Index Page
Activity: Herbicide
(Triazolopyrimidine)
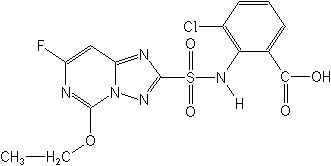
Structure for Cloransulam:
Adverse
Effects:
Blood
Body Weight Decrease
Cholesterol
Endocrine: Testicular
Endocrine: Thyroid
Kidney
Liver
Environmental
US:
As of February 17, 2005, US
tolerances for residues of Cloransulam-methyl and its acid,
Cloransulam are permitted on:
Soybean
(forage, hay, seed)
|
Blood
(click on for all fluorinated pesticides)
-- In the rat Chronic
Feeding / Carcinogenicity study the NOEL was equal to 75 mg/kg/day
and the Lowest Observed Effect Level (LOEL) was 325 mg/kg/day
based on significant increase in hemoglobin,
hematocrit, and red cell count in males, activities of
the liver enzymes aspartate and alanine
aminotransferase as well as alkaline phosphatase were decreased
in males, cholesterol was decreased in females, specific gravity
of urine was decreased in females, increased relative wight in
liver and relative weight of testes in males, males exhibited
an increased incidence of collecting duct hypertrophy and females
exhibited increased incidence of vacuolation in the kidney. There
was no evidence of carcinogenicity for cloransulam-methyl in this
study. In the mouse carcinogenicity study the NOEL was 10 mg/kg/day
and the LOEL was 108 mg/kg/day based on based on a decrease in
renal tubule vacuolation in male mice, increased size of centrilobular
and midzonal hepatocytes accompanied by altered tinctorial properties
in females and centrilobular hepatocyte hypertrophy in males.
Total tumor incidence (adenoma + carcinoma) was not increased
by dosing with cloransulam-methyl.
-- 21-day dermal (rabbit): Dermal Irritation NOEL 1000 mg/kg/day
Systemic NOEL = 500 mg/kg/day Systemic LOEL = 1000 mg/kg/day based
on decreased red cell count, hemoglobin
and hematocrit, anisocytosis and macrocytosis of red cells for
females.
-- Chronic Feeding/ Carcinogenicity (rat): NOEL = 75 mg/kg/day
LOEL = 325 mg/kg/day based on significant
increase in hemoglobin, hematocrit, and red cell count in males,
activities of the liver enzymes aspartate
and alanine aminotransferase as well as alkaline phosphatase were
decreased in males, cholesterol was decreased in females, specific
gravity of urine was decreased in females, increased relative
wight in liver and relative weight of testes in males, males exhibited
an increased incidence of collecting duct hypertrophy and females
exhibited increased incidence of vacuolation in the kidney. There
was no evidence of carcinogenicity for cloransulam-methyl
in this study.
Ref: USEPA. Pesticide Fact sheet. Cloransulam-methyl.
Reason for Issuance: Conditional Registration Date Issued: October
29, 1997. http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
... Cloransulam-methyl
84% DF was not found to be carcinogenic, teratogenic or to cause
reproductive effects. In-vitro and animal mutagenicity studies
were negative. Target organ effects have
been reported in the blood, kidney, liver,
testes and thyroid gland...
Ref: Material Safety Data Sheet for Gauntlet
Herbicide. MSDS Ref. No: F18-20-2. Date Approved: 09/25/2000.
Revision No. 1. FMC Corporation, Agricultural Products Group,
1735 Market Street, Philadelphia, PA 19103, USA.
Body
Weight Decrease (click
on for all fluorinated pesticides)
-- Developmental Toxicity
(rabbit): Maternal NOEL = 100 mg/kg/day Maternal LOEL = 300 mg/kg/day
based on reduced weight gain, food
efficiency, increased
abortions, and cesarean section observations. Developmental NOEL
= 300 mg/kg/day (HDT)
Ref: USEPA. Pesticide Fact sheet. Cloransulam-methyl.
Reason for Issuance: Conditional Registration Date Issued: October
29, 1997.
http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
-- In a two-year carcinogenicity study, XDE-565 (98.2% purity)
was administered to 60 B6C3F1 mice/sex/dose at dose levels of
0, 10, 100 or 1000 mg/kg bw/d in the diet for 24 months (with
an interim sacrifice of 10 mice/sex/dose at 12 months). No treatment-related
clinical signs were observed throughout the study duration. A
treatment-related effect on mean body-weight gain was observed.
On average, body-weight gains in high-dose males and mid- and
high-dose females were suppressed relative to controls... Based
on decreased body-weight gain in females
at $100 mg/kg bw/d, the NOAEL in male and female mice was determined
to be 10 mg/kg bw/d.
-- In a combined chronic and carcinogenicity study, XDE-565 (98.2%
purity) was administered to 60 Fischer 344 rats/sex/dose at 0,
10, 75 or 325 mg/kg bw/d in the diet for 24 months (with an interim
sacrifice of 10 rats/sex/dose at 12 months). Aside from an increase
in the incidence of perineal soiling in female rats at 75 and
325 mg/kg bw/d, no other treatment-related clinical findings were
observed. A treatment-related effect on mean body weight and body-weight
gain was observed. Relative to controls,
body weight and body-weight gain were suppressed in high-dose
males and females for a good portion of the dosing period.
Reference: Canadian Pest Management Regulatory
Agency. Regulatory Note REG2001-08.
http://www.hc-sc.gc.ca/pmra-arla/english/pdf/reg/reg2001-08-e.pdf
Cholesterol
(click on for all fluorinated
pesticides)
In the rat Chronic
Feeding / Carcinogenicity study the NOEL was equal to 75 mg/kg/day
and the Lowest Observed Effect Level (LOEL) was 325 mg/kg/day
based on significant increase in hemoglobin, hematocrit, and red
cell count in males, activities of the liver enzymes aspartate
and alanine aminotransferase as well as alkaline phosphatase were
decreased in males, cholesterol
was
decreased in females,
specific gravity of urine was decreased in females, increased
relative weight in liver and relative weight of testes in males,
males exhibited an increased incidence of collecting duct hypertrophy
and females exhibited increased incidence of vacuolation in the
kidney.
Ref: US EPA Fact Sheet. October 29, 1997.
http://www.fluoridealert.org/pesticides/cloransulam-methyl.epa.1997.htm
Endocrine:
Testicular (click
on for all fluorinated pesticides)
... Cloransulam-methyl
84% DF was not found to be carcinogenic, teratogenic or to cause
reproductive effects. In-vitro and animal mutagenicity studies
were negative. Target organ effects have
been reported in the blood, kidney, liver,
testes and thyroid gland...
Ref: Material Safety Data Sheet for Gauntlet
Herbicide. MSDS Ref. No: F18-20-2. Date Approved: 09/25/2000.
Revision No. 1. FMC Corporation, Agricultural Products Group,
1735 Market Street, Philadelphia, PA 19103, USA.
-- In the rat Chronic
Feeding / Carcinogenicity study the NOEL was equal to 75 mg/kg/day
and the Lowest Observed Effect Level (LOEL) was 325 mg/kg/day
based on significant increase in hemoglobin,
hematocrit, and red cell count in males, activities of the liver
enzymes aspartate and alanine aminotransferase as well as alkaline
phosphatase were decreased in males, cholesterol was decreased
in females, specific gravity of urine was decreased in females,
increased relative weight in liver
and relative weight of testes
in males...
-- Chronic Feeding/ Carcinogenicity (rat): NOEL = 75 mg/kg/day
LOEL = 325 mg/kg/day based on significant increase in hemoglobin,
hematocrit, and red cell count in males, activities of the liver
enzymes aspartate and alanine aminotransferase as well as alkaline
phosphatase were decreased in males, cholesterol was decreased
in females, specific gravity of urine was decreased in females,
increased relative weight in liver and
relative weight of testes in males...
Ref: USEPA. Pesticide Fact sheet. Cloransulam-methyl.
Reason for Issuance: Conditional Registration Date Issued: October
29, 1997.
http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
Endocrine:
Thyroid
(click
on for all fluorinated pesticides)
-- Cloransulam-methyl
was evaluated in 13-week dietary studies in rats, mice and dogs.
The primary target organs identified in these studies were the
kidneys (rat), the liver (mouse and dog), and thyroid
(rat). An NOEL was not determined in the rat based upon
minor histopathological changes in the kidney (males) and the
liver (females).
-- Chronic toxicity. In a 2-year combined chronic toxicity/ oncogenicity
study in the rat, the NOEL for chronic toxicity was 10 mg/ kg/day
based upon kidney and thyroid effects:
hypertrophy of collecting duct epithelial cells and vacuolation
consistent with fatty change in the proximal tubules of males
and females, and an increase in the incidence of mineralization
of the renal pelvis in males. Thyroid changes
were confined to the high dose males and consisted of hyperplasia
and hypertrophy of follicular epithelium.
Ref: Federal Register, March 26, 1997. DowElanco;
Pesticide Tolerance Petition Filing.
http://www.epa.gov/fedrgstr/EPA-PEST/1997/March/Day-26/p7496.htm
Kidney
(click on for all fluorinated
pesticides)
... Cloransulam-methyl
84% DF was not found to be carcinogenic, teratogenic or to cause
reproductive effects. In-vitro and animal mutagenicity studies
were negative. Target organ effects have
been reported in the blood, kidney,
liver, testes and thyroid gland...
Ref: Material Safety Data Sheet for Gauntlet
Herbicide. MSDS Ref. No: F18-20-2. Date Approved: 09/25/2000.
Revision No. 1. FMC Corporation, Agricultural Products Group,
1735 Market Street, Philadelphia, PA 19103, USA.
-- In the rat Chronic
Feeding / Carcinogenicity study the NOEL was equal to 75 mg/kg/day
and the Lowest Observed Effect Level (LOEL) was 325 mg/kg/day
based on significant increase in hemoglobin,
hematocrit, and red cell count in males, activities of the liver
enzymes aspartate and alanine aminotransferase as well as alkaline
phosphatase were decreased in males, cholesterol was decreased
in females, specific gravity of urine was decreased in
females, increased relative wight in liver
and relative weight of testes in males,
males exhibited an increased incidence of collecting duct hypertrophy
and females exhibited increased incidence of vacuolation in the
kidney. There was no evidence of carcinogenicity for cloransulam-methyl
in this study. In the mouse carcinogenicity study the NOEL was
10 mg/kg/day and the LOEL was 108 mg/kg/day based on based on
a decrease in renal tubule vacuolation in
male mice, increased size of centrilobular and midzonal
hepatocytes accompanied by altered tinctorial properties in females
and centrilobular
hepatocyte hypertrophy in males.
Total tumor incidence
(adenoma + carcinoma) was not increased by dosing with cloransulam-methyl.
-- Two Generation Reproduction (rat): Parental Systemic NOEL =
10 mg/kg/day Parental Systemic LOEL = 100 mg/kg/day based on
hypertrophy of the collecting ducts and vacuolation consistent
with fatty changes. Reproductive and Developmental NOEL = 100
mg/kg/day Reproductive and Developmental LOEL = 500 mg/kg/day
based on decreased live pups and increased pup deaths.
-- Chronic Feeding/ Carcinogenicity (rat):
NOEL = 75 mg/kg/day LOEL = 325 mg/kg/day based on significant
increase in hemoglobin, hematocrit, and red cell count in males,
activities of the liver enzymes aspartate and alanine aminotransferase
as well as alkaline phosphatase were decreased in males, cholesterol
was decreased in females, specific gravity of urine was
decreased in females, increased relative wight in liver
and relative weight of testes in males, males exhibited an increased
incidence of collecting duct hypertrophy and females exhibited
increased incidence of vacuolation in the kidney. There
was no evidence of carcinogenicity for cloransulam-methyl in this
study.
-- Carcinogenicity (mouse): NOEL = 10 mg/kg/day LOEL = 108 mg/kg/day
based on a decrease in renal tubule vacuolation
in male mice, increased size of centrilobular
and midzonal hepatocytes accompanied by altered tinctorial properties
in females and centrilobular hepatocyte hypertrophy in males.
Total tumor incidence (adenoma + carcinoma) was not increased
by dosing with cloransulam-methyl.
Ref: USEPA. Pesticide Fact sheet. Cloransulam-methyl.
Reason for Issuance: Conditional Registration Date Issued: October
29, 1997.
http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
Liver
(click
on for all fluorinated pesticides)
-- In a 90-day mouse
feeding study the NOEL was 50 mg/kg/day males. In a 21-day rabbit
dermal study the dermal irritation NOEL was equal or greater than
1000 mg/kg/day and the systemic NOEL was equal to 500 mg/kg/day.
In a 1-year dog chronic feeding study the NOEL was 10 mg/kg/day
and the LOEL was 50 mg/kg/day based on hepatocellular
hypertrophy and accumulation of pigment, and increased
activity of alkaline phosphatase and alanine aminotransferase
liver enzymes and decrease in albumin and total bilirubin.
-- In the rat Chronic Feeding / Carcinogenicity study the NOEL
was equal to 75 mg/kg/day and the Lowest Observed Effect Level
(LOEL) was 325 mg/kg/day based on significant
increase in hemoglobin, hematocrit, and red cell count in males,
activities of the liver enzymes aspartate
and alanine aminotransferase as well as alkaline phosphatase were
decreased in males, cholesterol was
decreased in females, specific gravity of urine was decreased
in females, increased relative wight in liver
and relative weight of testes in males,
males exhibited an increased incidence of collecting duct hypertrophy
and females exhibited increased incidence of vacuolation in the
kidney. There was no evidence of carcinogenicity for cloransulam-methyl
in this study. In the mouse carcinogenicity study the NOEL was
10 mg/kg/day and the LOEL was 108 mg/kg/day based on based on
a decrease in renal tubule vacuolation in
male mice, increased size of centrilobular and midzonal hepatocytes
accompanied by altered tinctorial properties in females and centrilobular
hepatocyte hypertrophy in males. Total tumor incidence (adenoma
+ carcinoma) was not increased by dosing with cloransulam-methyl.
-- 90-day dietary (mice): NOEL = 50 mg/kg/day (males) LOEL = 100
mg/kg/day for males based on increased levels of alkaline phosphatase
and increased liver weights and an
increase in the size of hepatocytes.
-- 1 Year Chronic Feeding (dog): NOEL = 10 mg/kg/day LOEL = 50
mg/kg/day based on hepatocellular hypertrophy
and accumulation of pigment, and increased activity of alkaline
phosphatase and alanine aminotransferase liver
enzymes and decrease in albumin and total bilirubin.
-- Chronic Feeding/ Carcinogenicity (rat): NOEL = 75 mg/kg/day
LOEL = 325 mg/kg/day based on significant
increase in hemoglobin, hematocrit, and red cell count in males,
activities of the liver enzymes aspartate
and alanine aminotransferase as well as alkaline phosphatase were
decreased in males, cholesterol was decreased
in females, specific gravity of urine was decreased in
females, increased relative weight in liver
and relative weight of testes in males, males exhibited an increased
incidence of collecting duct hypertrophy and females exhibited
increased incidence of vacuolation in the kidney.
There was no
evidence of carcinogenicity for cloransulam-methyl in this study.
-- Carcinogenicity (mouse): NOEL = 10 mg/kg/day LOEL = 108 mg/kg/day
based on a decrease in renal tubule vacuolation
in male mice, increased size of centrilobular and midzonal
hepatocytes accompanied by altered tinctorial properties in females
and centrilobular hepatocyte hypertrophy
in males. Total tumor incidence (adenoma + carcinoma) was
not increased by dosing with cloransulam-methyl.
Ref: USEPA. Pesticide Fact sheet. Cloransulam-methyl.
Reason for Issuance: Conditional Registration Date Issued: October
29, 1997.
http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
... Cloransulam-methyl
84% DF was not found to be carcinogenic, teratogenic or to cause
reproductive effects. In-vitro and animal mutagenicity studies
were negative. Target organ effects have
been reported in the blood, kidney, liver,
testes and thyroid gland...
Ref: Material Safety Data Sheet for Gauntlet
Herbicide. MSDS Ref. No: F18-20-2. Date Approved: 09/25/2000.
Revision No. 1. FMC Corporation, Agricultural Products Group,
1735 Market Street, Philadelphia, PA 19103, USA.
| Environmental
(click on for all fluorinated
pesticides)
--
The structurally-similar transformation products occur in
aerobic and anaerobic metabolism studies and appear persistent
under anaerobic conditions. Cloransulam-methyl is
highly mobile while the major transformation product also
appears to be mobile in soils. Cloransulam-methyl
dissipated relatively rapidly from the upper 15 cm of bare
ground plots. Transformation products indicate that metabolism
and photolysis are likely to be major routes of transformation
of cloransulam-methyl in the field. Leaching
may play an important role in dissipation of cloransulam-methyl
from the surface layer. So while cloransulam-methyl and
its transformation products are likely to be only of slight
persistence in the surface, the chemicals
may become more persistent when leached into the subsurface.
A label Groundwater Application Advisory is required and
prospective groundwater monitoring studies are a condition
of registration.
-- Due to concerns about the mobility and potential persistence
of cloransulam-methyl and its structurally-similar, biologically
active transformation products in the subsurface and ground
water, and surface water the following label statements
are required: Groundwater Advisory This chemical and its
transformation products demonstrate the properties and characteristics
associated with chemical detected in ground water. The use
of this chemical in areas where soils are permeable particularly
where the water table is shallow, may
result in ground-water contamination. Surface Water
Advisory This chemical can contaminate surface water through
spray drift.
-- Under some conditions, this chemical, and/or its transformation
products, may have a high
potential for runoff into surface water (primarily via dissolution
in run off water) for several weeks post-application.
Vulnerable Conditions include poorly draining or
wet soils with readily visible slopes toward adjacent surface
waters, frequently flooded area, areas over-laying extremely
shallow ground water, areas with in-field canals or ditches
that drain to surface-water, areas not separated or adjacent
surface waters with vegetated filter strips, and areas over-laying
tile drainage systems that drain to surface water.
-- Prospective groundwater monitoring studies are a condition
of registration. The Health Level in water for cloransulam-methyl
and/or its acid, cloransulam, is 1000 ppb based on an RfD
of 0.1 mg/kg bw/day, and a 10 kg child consuming 1 Liter
of water of day. An Ecotoxicity Level will be determined,
if necessary, upon receipt and review of the Tier II Phytotoxicity
Studies required as a condition of registration. DowElanco
agreed to exposure reductions if residues of cloransulam-methyl
occur at or above 50% of the health level (500 ppb) in public
and private wells. DowElanco will develop modeling scenarios
for each of the prospective groundwater monitoring sites
simulating conditions over a maximum of 100 years.
Ref: USEPA. Pesticide Fact
sheet. Cloransulam-methyl. Reason for Issuance: Conditional
Registration Date Issued: October 29, 1997.
http://www.epa.gov/opprd001/factsheets/cloransulam.pdf
|
| A
February
17, 2005,
check at the Code
of Federal Regulations for Cloransulam-methyl and its acid Cloransulam:
this herbicide is permitted in
or on 3 food
commodities in the United States.
The
following list identifies these crops for which EPA has set
pesticide tolerances.
|
[Code
of Federal Regulations]
[Title 40, Volume 22]
[Revised as of July 1, 2004]
From the U.S. Government Printing Office via GPO Access
[CITE: 40CFR180.514]
[Page 488]
TITLE 40--PROTECTION OF ENVIRONMENT
CHAPTER I--ENVIRONMENTAL PROTECTION AGENCY (CONTINUED)
PART 180_TOLERANCES AND EXEMPTIONS FROM TOLERANCES FOR PESTICIDE
CHEMICALS
IN FOOD--Table of Contents
Subpart C_Specific Tolerances
Sec. 180.514 Cloransulam-methyl;
tolerances for residues.
(a) General. Tolerances are established
for residues of the
herbicide, cloransulam-methyl, N-(2-carboxymethyl-6-chlorophenyl)-5-
ethoxy-7-fluoro-(1,2,4)-triazolo[1,5c]-pyrimidine-2-sulfonamide,
plus
its acid, cloransulam, calculated as parent ester in
or on the following
raw agricultural commodities: |
Commodity |
As
of
September 25,
2003
PPM |
As
of
February 17,
2005
PPM |
| Soybean,
forage |
0.1 |
0.1 |
| Soybean,
hay |
0.2 |
180.514
|
| Soybean,
seed |
0.02 |
180.514
|
(b) Section 18 emergency exemptions. [Reserved]
(c) Tolerances with regional registrations. [Reserved]
(d) Indirect or inadvertent residues. [Reserved] |
|

